Abstract
Fibronectin-binding proteins mediate Staphylococcus aureus internalization into nonphagocytic cells in vitro. We have investigated whether fibronectin-binding proteins are virulence factors in the pathogenesis of pneumonia by using S. aureus strain 8325-4 and isogenic mutants in which fibronectin-binding proteins were either deleted (DU5883) or overexpressed [DU5883(pFnBPA4)]. We first demonstrated that fibronectin-binding proteins mediate S. aureus internalization into alveolar epithelial cells in vitro and that S. aureus internalization into alveolar epithelial cells requires actin rearrangement and protein kinase activity. Second, we established a rat model of S. aureus-induced pneumonia and measured lung injury and bacterial survival at 24 and 96 h postinoculation. S. aureus growth and the extent of lung injury were both increased in rats inoculated with the deletion mutant (DU5883) in comparison with rats inoculated with the wild-type (8325-4) and the fibronectin-binding protein-overexpressing strain DU5883(pFnBPA4) at 24 h postinfection. Morphological evaluation of infected lungs at the light and electron microscopic levels demonstrated that S. aureus was present within neutrophils from both 8325-4- and DU5883-inoculated lungs. Our data suggest that fibronectin-binding protein-mediated internalization into alveolar epithelial cells is not a virulence mechanism in a rat model of pneumonia. Instead, our data suggest that fibronectin-binding proteins decrease the virulence of S. aureus in pneumonia.
In a recent survey, Staphylococcus aureus was found to be the most common cause of lower respiratory tract infections in Europe, the United States, Canada, Latin America, and the Western Pacific region (9). In view of the fact that S. aureus infections are increasingly difficult to treat because of the high percentage of antibiotic-resistant strains (34), a better understanding of the molecular basis of S. aureus virulence in pneumonia may help in the design of new therapeutic strategies.
S. aureus has long been regarded as an extracellular pathogen because it is rarely observed inside cells in vivo and because it secretes a range of toxins that are cytolytic to many host cell types (14, 29). However, recent in vitro studies demonstrate that S. aureus is internalized and survives inside nonphagocytic cells (1, 2, 12, 18, 19, 21). Fibronectin-binding proteins present on the surface of S. aureus (16, 19, 26) mediate internalization into nonphagocytic cells. S. aureus fibronectin-binding proteins bind β1-integrins on the surface of the host cells by means of a fibronectin bridge (16).
Survival of internalized S. aureus within nonphagocytic cells may be an additional virulence mechanism in S. aureus infections (20). Internalized S. aureus may be able to evade or delay elimination by the host's immune system and avoid extracellular antibiotics (20). If internalization contributes to S. aureus persistence in vivo, then drugs which interfere with fibronectin binding to host cell integrins may have a role to play in treatment of S. aureus infections (6).
Alveolar epithelial type I cells are large squamous cells that cover over 95% of the lungs' surface area; the remaining 5% is covered by alveolar epithelial type II cells. Both alveolar epithelial type I and II cells have a number of potential fibronectin-binding receptors on their cell surfaces (7, 28, 32). The overall objective of our study was to investigate whether fibronectin-binding protein-mediated internalization into alveolar epithelial cells is a virulence mechanism in S. aureus-induced pneumonia.
MATERIALS AND METHODS
All reagents and materials were supplied by Sigma, Dorset, United Kingdom, unless stated otherwise.
Bacteria.
The S. aureus strains used in this study are derivatives of the wild-type strain 8325-4. Strain DU5883 is an isogenic mutant of strain 8325-4 disrupted in the fnbA (fnbA::Tcr) and fnbB (fnbB::Emr) genes (17). DU5883(pFnBPA4) is the deletion mutant with fnbA expressed on a high copy plasmid (17). All strains were grown overnight in Todd Hewitt broth (B. D. Biosciences, Oxford, United Kingdom); DU5883(pFnBPA4) was selected with 10 μg of chloramphenicol per ml. The identity of each S. aureus strain was regularly checked by using antibiotic disks (B. D. Biosciences). Overnight cultures were washed twice with endotoxin-free phosphate-buffered saline (PBS) before resuspension in PBS for all experiments.
Alveolar epithelial cell line.
Simian virus 40 (SV40)-transformed strain AT2 neonatal alveolar epithelial cells were used for the in vitro internalization assays (4). SV40-AT2 cells retain the sodium transport properties of alveolar type II cells and express RTI40 (rat alveolar epithelial type I cell protein; molecular mass, approximately 40 kDa) (25, 31). SV40-AT2 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Labtech International, East Sussex, United Kingdom), penicillin (100 U/ml), and streptomycin sulfate (100 μg/ml) (Invitrogen, Paisley, United Kingdom). SV40-AT2 cells were maintained at 37°C in a 5% CO2 humidified incubator (31). SV40-AT2 cells were prepared for internalization assays by seeding semiconfluent 75-cm2 flasks onto six-well plates (Fred Baker Scientific, Cheshire, United Kingdom) and incubating overnight in DMEM plus 10% FCS.
Control SV40-AT2 cells were checked during the experimental period for mycoplasmas by immunofluorescence staining with Hoechst 33258 DNA-binding dye (only the SV40-AT2 nuclei stained with the DNA-binding dye in mycoplasma-negative cells) (38).
Internalization assay.
Confluent SV40-AT2 cells were incubated for 1 h in serum-free DMEM and then washed twice in PBS with calcium and magnesium. DMEM (1 ml) containing 106 CFU of S. aureus per ml was added to each well that contained SV40-AT2 cells (approximately 1.5 × 106 SV40-AT2 cells per well). S. aureus was cocultured with SV40-AT2 cells for 2 to 6 h. At the end of the coculture period, SV40-AT2 cells were washed twice with PBS and then incubated for 1 h in the presence of gentamicin (100 μg/ml in serum-free DMEM). The SV40-AT2 cells were then washed three times with PBS and lysed with 1% (wt/vol) NP-40 (ICN Biomedicals, Basingstoke, United Kingdom) in 10 mM Tris-HCl buffer, pH 8.0, containing 154 mM NaCl and complete protease inhibitor cocktail (Roche Diagnostics, East Sussex, United Kingdom). The cell lysate was diluted and plated out in triplicate on tryptic soy agar (TSA) plates supplemented with sheep blood (B. D. Biosciences). The plates were incubated overnight at 37°C, and the number of CFU was recorded to determine the number of intracellular S. aureus. S. aureus CFU were only recovered in the presence of SV40-AT2 cells.
Effects of cytochalasin D and genistein.
The cytochalasin D and genistein assay was a modification of the internalization assay described above. SV40-AT2 cells were incubated for 1 h at 37°C with either cytochalasin D (2 μM) or genistein (250 μM) (Roche Diagnostics) (18). Internalization assays were then performed in the presence of inhibitors for 2 h at 37°C. At the end of the experimental period, SV40-AT2 cells were washed and incubated with gentamicin. In some experiments, washed SV40-AT2 cells were reincubated with S. aureus for an additional 2 or 4 h to demonstrate that cytochalasin D and genistein were not toxic to SV40-AT2 cells. The effects of cytochalasin D and genistein on the number of internalized S. aureus were compared to the extent of S. aureus internalized in the absence of inhibitors.
Pneumonia model.
Male specific-pathogen-free rats (300 to 350 g) were obtained from Harlan United Kingdom, Ltd. (Oxton, United Kingdom). Rats were anaesthetized with an intraperitoneal injection of 1 ml of a mixture of Hypnorm (0.315 mg of fentanyl citrate and 10 mg of fluanisone per ml) and Hypnoval (5 mg of midazolam hydrochloride per ml) (40). The rat's mouth was opened, and the trachea was visualized with the aid of a laryngoscope. A catheter (0.96 cm external diameter, 9 cm in length) (Portex fine bore polyethylene tubing; Portex Ltd., Kent, United Kingdom) was inserted into the distal airways. S. aureus (approximately 108 CFU/0.5 ml) was instilled into the distal airways down the catheter. PBS was instilled into the distal airways of control rats.
Experimental design.
Lung tissue was obtained from rats inoculated with 8325-4 (n = 3), DU5883 (n = 3), or PBS (vehicle) (n = 3) at 24 h postinfection for analysis at the light microscopic level. For morphological analysis of lung tissue at the electron microscopic level, lung tissue was obtained from rats inoculated with 8325-4 (n = 2), DU5883 (n = 2), and PBS (n = 2) at 24 h postinfection. Bronchoalveolar lavage (BAL) fluid and lung tissue were obtained from rats inoculated with 8325-4 at 24 h (n = 7) and 96 h (n = 5) postinfection; from rats inoculated with DU5883 at 24 h (n = 7) and 96 h (n = 6) postinfection; and from rats inoculated with DU5883(pFnBPA4) at 24 h (n = 7) and 96 h (n = 5) postinfection. Control rats (PBS instilled) were also included at 24 h (n = 7) and 96 h (n = 5). All animal experiments were carried out according to the Animals (Scientific Procedures) Act, 1986.
Collection of lung tissue, blood, and BAL fluid samples.
At the end of the experimental period, rats were anaesthetized with pentobarbital (45 mg/kg intraperitoneally) containing heparin (500 U/kg intraperitoneally) (22-24). Blood was collected from the ascending aorta. The trachea was canulated, and the lungs were lavaged two times with sterile PBS. The lungs were then removed. BAL fluid was centrifuged at 900 × g for 5 min (23, 24). BAL fluid leukocytes were quantified by hemacytometric counting. Differential cell counts on BAL fluid cells were performed on cytocentrifuged preparations fixed with methanol and stained with Diff-Quick. A total of 300 cells were counted per cytospin.
Protein assay.
BAL fluid protein concentration was determined by using the Bio-Rad assay (Bio-Rad Laboratories, Hertfordshire, United Kingdom) with bovine serum albumin as a standard (Pierce Warriner, Chester, United Kingdom) (24). Data are expressed as the total amount of protein recovered in BAL fluid (i.e., milligrams of protein per milliliter multiplied by the total volume of BAL fluid recovered).
RTI40 assay.
The amount of RTI40 (a biochemical marker for type I cell damage) was measured in an enzyme-linked immunosorbent assay (ELISA)-based assay essentially as described previously (22, 23). RTI40 was assayed on the insoluble fraction of BAL fluid (500,000 × gav for 10 min in a Beckman Optima ultracentrifuge). Data are calculated as the total amount of RTI40 recovered in BAL fluid (i.e., relative densitometry units of RTI40 per milliliter multiplied by the total volume of BAL fluid) and then expressed as a percentage of the control values.
Lung homogenates.
Lungs were weighed and homogenized in Tris-HCl (2.42 mM)-buffered saline (TBS), pH 8.2, containing NaCl (154 mM) and proteinase inhibitor cocktail (Roche Diagnostics). Lungs were homogenized by using a blender (Ultra Turrax Y25; IKA-Werke) on ice at approximately 4,000 rpm twice for 5 s each. Lung homogenates were serially diluted and plated out on blood agar plates to determine the CFU.
Immunofluorescence analysis.
Lungs were fixed with paraformaldehyde (4% [wt/vol] in PBS) to determine the extent of alveolar epithelial necrosis in S. aureus pneumonia at 24 h postinfection (24). Frozen lung sections (3 μm) were incubated with monoclonal antibodies (MAb) against alveolar epithelial type I cells (anti-RTI40 MAb) (10) and two different MAb against alveolar epithelial type II cells (anti-MMC4 antigen and anti-RTII70) (3) (11), followed by isotype-specific secondary antibodies. Fluorescein isothiocyanate (FITC) conjugated to anti-immunoglobulin G1 (IgG1) was used to detect the RTI40 protein (Lorne Laboratories, Reading, United Kingdom) (3), Rhodamine-conjugated to anti-IgG2a (Lorne Laboratories) was used to detect the MMC4 protein (3), and Alexa Fluor 350 conjugated to anti-IgG3 was used to detect the anti-RTII70 antigen (Molecular Probes, Lieden, Netherlands). The nucleus was stained with the DNA-binding dye Hoechst 33258. Immunofluorescence-stained sections were observed by epifluorescence illumination (Axiovert S100; Zeiss). Images were captured with a Cool Snap camera, and the images were processed by using Improvision OpenLab software (version 2.2).
Gram stain.
Paraffin sections were Gram stained to visualize S. aureus in lung tissue at 24 h postinfection. Cytospun cells were also Gram stained to identify S. aureus in neutrophils and macrophages from BAL fluid.
Electron microscopic analysis.
Tissue samples for electron microscopy were selected from the hemorrhaged region of lungs inoculated with either 8325-4 or DU5883. Similar regions were taken from control lungs. Lung blocks were fixed with glutaraldehyde buffer (3%) containing sodium cacodylate (0.1 M). Lung blocks were postfixed in osmium tetroxide buffer (1%) containing sodium cacodylate (0.1 M). Lung blocks were then dehydrated in a graded series of alcohol and embedded in polyethylene capsules in fresh Araldite epoxy resin. Lung sections were cut and placed on 200-mesh uncoated grids and then stained with uranyl acetate and lead citrate for electron microscopic studies. Sections were viewed with a Philips CM12 electron microscope.
Statistical analysis.
All data are expressed as means ± standard error of the mean. Data from the in vitro internalization assays were transformed to reflect the total number of S. aureus recovered per well or transformed to fractions of control values. Multiple sample means were compared by using one-way analysis of variance with the Student-Newman-Keuls posttest.
Multiple sample means from data generated from the pneumonia models (CFU, leukocyte numbers, and BAL protein and RTI40 concentrations) were compared by using Kruskal-Wallis analysis of variance (nonparametric analysis of variance), with Dunn's multiple comparison posttest. P < 0.05 was considered significant. Tests were performed by using GraphPad InStat v3.00 for Windows 95 (www.graphpad.com).
RESULTS
Fibronectin-binding proteins are important for the internalization of S. aureus by SV40-AT2 cells.
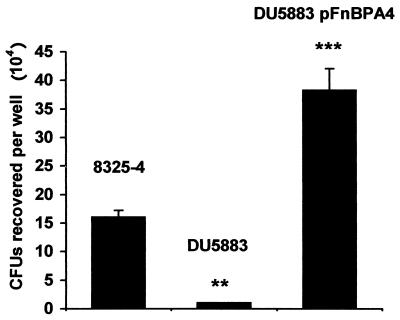
The presence or absence of fibronectin-binding proteins (Fig. 1) significantly affected the extent of S. aureus internalization into SV40-AT2 cells. DU5883, which does not express either FnBPA or -B, was internalized 20-fold less than 8325-4 (P < 0.01). DU5883(pFnBPA4), which overexpresses FnBPA, was internalized 2.4-fold more than 8325-4 (P < 0.001) (Fig. 1). These data demonstrate that fibronectin-binding proteins are important for internalization of S. aureus into rat alveolar epithelial cells.
FIG. 1.
Effect of fibronectin-binding proteins on S. aureus internalization into alveolar epithelial cells. SV40-AT2 cells were cocultured with either 8325-4, DU5883, or DU5883(pFnBP4) for 6 h. Extracellular S. aureus cells were killed with gentamicin. The experiment was performed three times; data from one representative experiment are shown. ∗∗, P < 0.01 versus control; ∗∗∗, P < 0.001 versus control.
SV40-AT2 intracellular traffic is important for internalization of S. aureus.
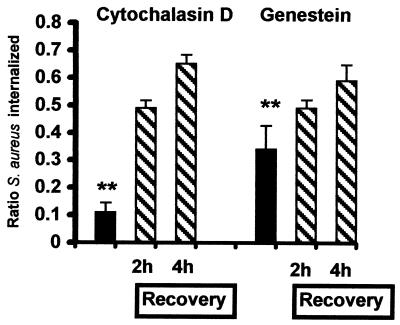
S. aureus strain 8325-4 was cocultured with SV40-AT2 cells for 2 h in the presence of inhibitors of intracellular traffic (Fig. 2). Cytochalasin D, an inhibitor of actin polymerization, reduced the extent of S. aureus internalization by 91% (Fig. 2). Genistein, a protein tyrosine kinase inhibitor, reduced internalization by 74% (P < 0.01) (Fig. 2). The inhibitors were not toxic to SV40-AT2 cells, since the extent of internalization increased following the removal of these inhibitors from the incubation medium (Fig. 2).
FIG. 2.
Effects of cytochalasin D and genistein on S. aureus internalization into alveolar epithelial cells. SV40-AT2 cells were cocultured with 8325-4 in the presence of inhibitors for 2 h (black bars). SV40-AT2 cells were then washed and reincubated for an additional 2 h or 4 h (diagonal stripes) in the presence of only S. aureus. The extent of internalization was compared at each time point with that of SV40-AT2 cells cultured with S. aureus alone. Data are presented as the ratio of S. aureus internalized in the presence of inhibitor to S. aureus internalized in the absence of inhibitor. The experiment was performed twice with similar results. Data from one experiment are shown. Both inhibitors significantly reduced the extent of 8325-4 internalization into SV40-AT2 cells in comparison to SV40-AT2 cells cocultured with just 8325-4. ∗∗, P < 0.01 versus SV40-AT2 cells cocultured with 8325-4.
Morphological changes to the lung in 8325-4-infected rats.
Distal airway instillation of 8325-4 into rat lungs induced pneumonia in the left lung in the majority of experiments. At autopsy, the involved lobe turned from red at 24 h postinfection to grey at 96 h postinfection. Light microscopic analysis of lung tissue at 24 h postinfection demonstrated that leukocytes had filled the distal air spaces (Fig. 3C).
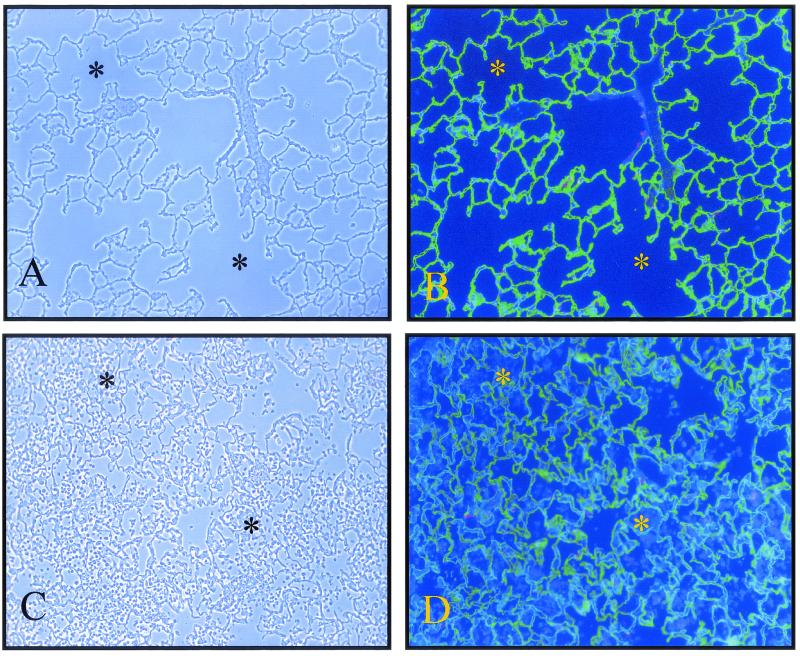
FIG. 3.
Immunofluorescence visualization of leukocyte influx and alveolar epithelium in S. aureus 8325-4-inoculated lungs at 24 h postinfection. Alveolar epithelial type I cells were stained with the anti-RTI40 MAb (green), while alveolar epithelial type II cells were stained with the MMC4 MAb (red). Nuclei were stained with the Hoechst DNA-binding dye. (A) Phase-contrast image of control lung. (B) Corresponding immunofluorescence image. (C) Phase-contrast image of 8325-4-inoculated lung. (D) Corresponding immunofluorescence image. Many of the air spaces (stars) are filled with leukocytes ((Hoechst 33258-only-positive cells) in 8325-4-inoculated lungs in comparison with control lungs. Original magnification, ×100.
The morphological integrity of alveolar epithelial type I and II cells in 8325-4-infected lungs was determined by immunofluorescence analysis with the aid of cell-selective antibodies (3, 10, 11). Type I cells were visualized with the aid of an anti-RTI40 MAb (green staining) (Fig. 3 and 4). In control lungs, the anti-RTI40 MAb bound to the apical surface of alveolar epithelial type I cells (Fig. 3 and 4). In 8325-4-inoculated lungs, the pattern and extent of anti-RTI40 binding were similar to those in control lungs in most regions of the lung (Fig. 3 and 4).
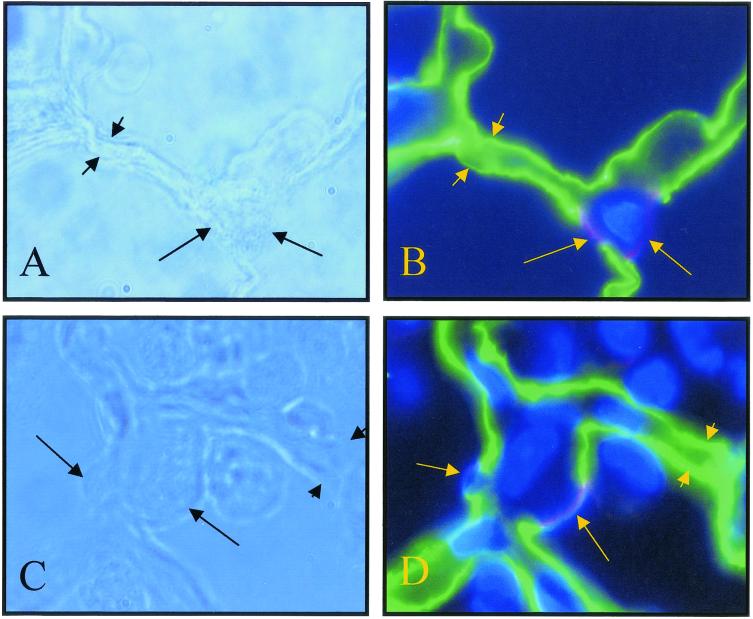
FIG. 4.
High-magnification view of alveolar epithelium in S. aureus 8325-4-inoculated lungs at 24 h postinfection. Alveolar epithelial type I cells were stained with the anti-RTI40 MAb (green, short arrows), and alveolar type II cells were stained with the MMC4 and anti-TII70 cell MAbs (red and blue, respectively, long arrows). Nuclei were stained with the Hoechst DNA-binding dye. (A) Phase-contrast image of control lung. (B) Corresponding immunofluorescent image. (C) Phase-contrast image of 8325-4-inoculated lung. (D) Corresponding immunofluorescent image. Binding of alveolar cell-selective antibodies in 8325-4-infected lungs was not dissimilar from that in control lungs despite the ongoing inflammation. Original magnification, ×1,000.
Alveolar epithelial type II cells were identified by using two different MAbs. The anti-RTII70 MAb recognizes the apical surface of type II cells (blue apical staining) (Fig. 4) (11), while the MMC4 MAb recognizes the apical surface of alveolar epithelial type II and Clara cells (red staining) (Fig. 4) (3). The pattern and extent of both anti-type II MAb binding in 8325-4-infected lungs 24 h postinfection were comparable to that in control sections (Fig. 4). Occasionally, MMC4- and RTII70-positive cells (type II cells) were rounded-up and appeared to be in the process of shedding from their basement membranes in 8325-4-infected lungs at 24 h postinfection (data not shown).
Effect of fibronectin-binding proteins on recovery of S. aureus in lung tissue and BAL fluid.
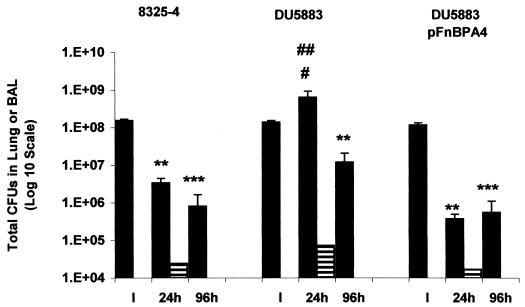
The number of CFU recovered in lung tissue 24 h postinfection decreased by over 97% in rats inoculated with either 8325-4 or DU5883(pFnBPA4) in comparison to the number of CFU instilled (Fig. 5). In contrast, the number of CFU recovered in lung tissue from DU5883-inoculated rats was increased by 4.6-fold relative to the instillate at 24 h postinfection (Fig. 5). Moreover, the number of CFU recovered in DU5883-infected lungs at 24 h was increased by over 190-fold relative to the number in 8325-4-infected rats (P < 0.05) and by 1,700-fold (P < 0.01) in comparison to the number of CFU recovered from DU5883(pFnBPA4)-infected lungs (Fig. 5). By 96 h postinfection, the number of CFU recovered from DU5883-inoculated lungs was approaching the number of CFU obtained from both 8325-4- and DU5883(pFnBPA4)-inoculated lungs (Fig. 5).
FIG. 5.
Number of viable S. aureus recovered in lung tissue and BAL fluid at 24 and 96 h postinfection. The number of viable S. aureus (CFU) was determined in lung tissue (black bars) and BAL fluid (horizontal bars). ∗∗, P < 0.01 versus instillate CFU; ∗∗∗, P < 0.001 versus instillate CFU; #, P < 0.05 versus 8325-4; ##, P < 0.01 versus DU5883(pFnBPA4).
In contrast to the lung data, the number of CFU recovered in BAL fluid at 24 h was not significantly different between the three S. aureus strains (Fig. 5). No CFU were recovered in BAL fluid at 96 h postinfection in any of the S. aureus-infected lungs.
Effect of fibronectin-binding proteins on leukocyte recruitment to the lungs in pneumonia.
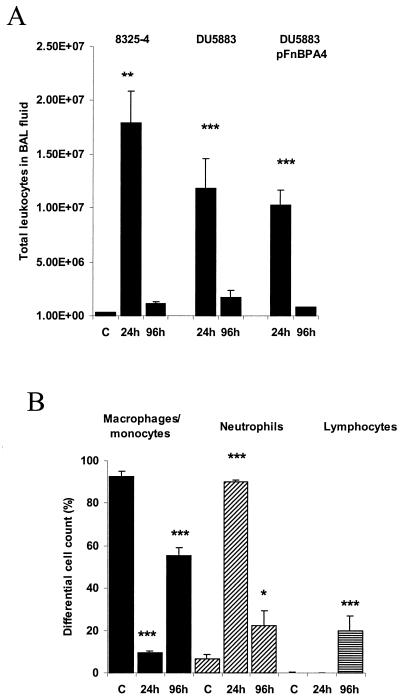
The total number of leukocytes recovered in BAL fluid from 8325-4-infected rats was elevated 57-fold above control values at 24 h (Fig. 6A). At 24 h postinfection, most of the leukocytes in BAL fluid from 8325-4-infected rats were neutrophils (90%) (Fig. 6B). However, by 96 h postinfection the percentage of neutrophils had decreased, while that of both monocytes/macrophages and lymphocytes had increased (Fig. 6B).
FIG. 6.
Number and profile of leukocytes recovered in BAL fluid from S. aureus-inoculated lungs at 24 and 96 h postinfection. (A) Total number of leukocytes recovered in BAL fluid from 8325-4-, DU5883- and DU5883(pFnBPA4)-inoculated lungs. (B) Percentage of neutrophils (black bars), macrophages and monocytes (diagonally striped bars), and lymphocytes (horizontally striped bars) in BAL fluid from control and 8325-4-inoculated lungs. ∗, P < 0.05 versus control; ∗∗, P < 0.01 versus control; ∗∗∗, P < 0.001 versus control.
The total number of leukocytes recovered in BAL fluid from DU5883- and DU5883(pFnBPA4)-infected rats was significantly elevated over control values, by 37-fold and 33-fold, respectively (Fig. 6A). However, there were no significant differences in the total number of leukocytes in BAL fluid between the different strains at either 24 or 96 h postinfection. Nor was the percentage of neutrophils, macrophages/monocytes, and lymphocytes recovered in BAL fluid significantly different between S. aureus strains at either 24 or 96 h postinfection (data not shown).
Damage to the air-blood barrier in S. aureus pneumonia.
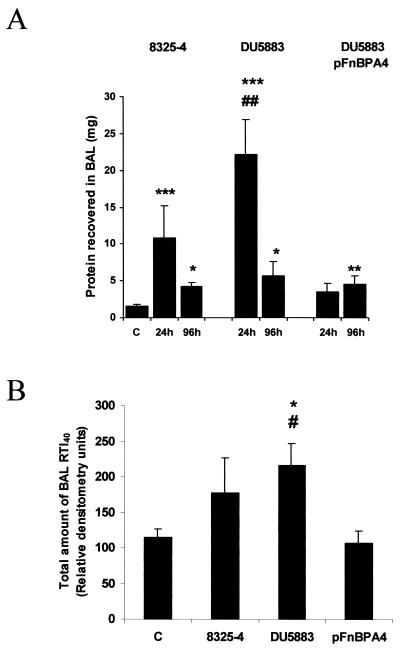
The amount of protein recovered in BAL fluid from 8325-4-infected rats was elevated by 6.8-fold above control values at 24 h postinfection but by only 2.7-fold at 96 h postinfection (Fig. 7A). The amount of protein recovered in BAL fluid from DU5883-infected rats was significantly elevated over values from both control and DU5883(pFnBPA4)-inoculated rats at 24 h postinfection (Fig. 7A). However, by 96 h postinfection, the amount of protein recovered in BAL fluid was not significantly different between the three different strains (Fig. 7A).
FIG. 7.
Amount of protein and RTI40 recovered in BAL fluid at 24 and 96 h postinfection. (A) The amount of protein recovered in BAL fluid from 8325-4-, DU5883-, and DU5883(pFnBPA4)-inoculated rats at 24 and 96 h postinfection. (B) The amount of RTI40 recovered in BAL fluid at 24 h postinfection. ∗, P < 0.05 versus control; ∗∗, P < 0.01 versus control; ∗∗∗, P < 0.001 versus control; #, P < 0.05 versus DU5883(pFnBPA4); ##, P < 0.01 versus DU5883(pFnBPA4).
To determine the extent of alveolar epithelial type I cell necrosis in S. aureus pneumonia, the amount of RTI40 was also measured in BAL fluid. The amount of RTI40 recovered in BAL fluid from 8325-4- and DU5883(pFnBPA4)-infected rats was not significantly different from control values (Fig. 7B). However, the amount of RTI40 recovered from rats inoculated with DU5883 was significantly elevated over both control and DU5883(pFnBPA4)-infected rats at 24 h postinfection (Fig. 7B). There were no differences in the amount of RTI40 recovered in BAL fluid from rats inoculated with S. aureus at 96 h postinfection (data not shown).
Cellular location of S. aureus in 8325-4- and DU5883-infected lungs.
Gram stains of both BAL fluid cells (cytospins) and infected lung tissue (paraffin sections) demonstrated that S. aureus was predominantly associated with neutrophils from infected rats (data not shown). In addition, the percentage of neutrophils containing one or more Gram stain particles was not different between 8325-4- and DU5883-infected lungs (10.3 ± 1.45 versus 11.8 ± 6.08, respectively). Macrophages occasionally contained a few gram-positive particles in their cytoplasm. Gram-positive particles were very occasionally associated with the alveolar wall in both 8325-4- and DU5883-infected lungs (data not shown). However, it was not possible to resolve the location of the gram-positive particles by light microscopy.
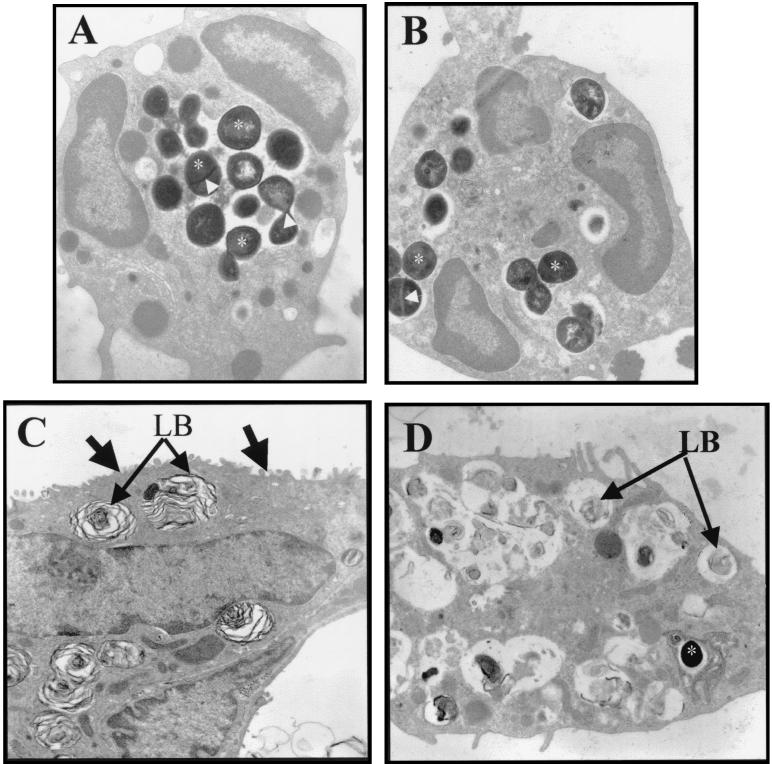
Electron microscopic analysis confirmed that neutrophils from both 8325-4- and DU5883-infected lungs contained S. aureus (Fig. 8A and B). Electron microscopic analysis of lung tissue failed to demonstrate any S. aureus internalized with alveolar epithelial cells in 8325-4-inoculated rats. However, S. aureus was observed in one necrotic alveolar epithelial type II cell from DU5883-inoculated rats (Fig. 8C and D).
FIG. 8.
Cellular location of S. aureus at the electron microscopic level in 8325-4- and DU5883-inoculated rats. (A and B) Electron micrograph of neutrophils from 8325-4-infected (A) and DU5883-infected (B) lungs. S. aureus (electron dense, approximately 1-μm-diameter particles) cells are internalized into neutrophils from both 8325-4- and DU5883-infected lungs (stars on some internalized S. aureus). The electron-dense line across some S. aureus (arrows in A and B) is a cell wall associated with cell division (37). (C and D) Electron micrograph of an alveolar epithelial type II cell from control (C) and DU5883-inoculated (D) lungs. Type II cell from control lung contains characteristic lamellar bodies (LB) and apical microvilli (arrows) (C). Type II cell from DU5883-infected lung has sloughed from its basement membrane and contains remnants of lamellar bodies (LB) (D). One S. aureus bacterium is located in a vacuole within the cytoplasm of the sloughed type II cell (star) (D). Original magnification: A and B, ×13,000; C and D, ×8,000.
DISCUSSION
We have demonstrated that S. aureus is internalized into rat alveolar epithelial cells in vitro and that internalization is mediated by fibronectin-binding proteins located on the surface of the bacteria. S. aureus internalization into alveolar epithelial cells also requires an active cytoskeleton, since internalization was inhibited by cytochalasin D, which disrupts actin structures. Furthermore, our data suggest that S. aureus internalization is a regulated process, since the inhibition of tyrosine kinases by genistein prevented uptake. Similar results have been reported previously for S. aureus internalization into other nonprofessional phagocytic cells, including mammary gland epithelial cells, endothelial cells, and osteoblasts (1, 12, 15, 18, 24, 26).
To determine whether the expression of fibronectin-binding proteins contributes to virulence in vivo, we first established a rat model of S. aureus-induced pneumonia (strain 8325-4). Instillation of S. aureus (108 CFU) into the distal airways of rat lungs induced an acute inflammatory reaction that was resolving by 96 h postinfection. The total number of leukocytes and the percentage of neutrophils in BAL fluid were both elevated at 24 h postinfection but had returned to near control values at 96 h postinfection. Similarly, the amount of protein recovered in BAL fluid, which is a measure of serum flux into the air spaces, was elevated at 24 h but returned to near control levels at 96 h postinfection. However, despite the elevated number of leukocytes and protein in BAL fluid, our data did not reveal major necrosis of alveolar epithelial type I cells in 8325-4-induced pneumonia.
To determine whether fibronectin-binding proteins contribute to S. aureus virulence in pneumonia, we compared the survival of three S. aureus strains. These three strains were the wild-type 8325-4, a mutant (DU5883) in which both the fnbPA and fnBPB genes were deleted, and the same mutant containing the gene for FnBPA on a high-copy-number plasmid [DU5883(pFnBPA4)] (17). Contrary to what we expected, the number of viable DU5883 cells in lung tissue at 24 h postinfection was 190-fold greater than the number of 8325-4 cells and 1,700-fold greater than the number of DU5883(pFnBPA4) cells. However, by 96 h postinfection, the number of viable DU5883 S. aureus had declined to values approaching those of both the wild type and the complemented mutant. These data suggest that fibronectin-binding proteins promote S. aureus elimination from the lungs.
The extent of acute lung injury, as measured by the total amount of protein and RTI40 recovered in BAL fluid, was also increased at 24 h postinfection in DU5883-infected rats in comparison with values obtained from rats inoculated with either the parental 8325-4 or the complemented mutant strain. The amount of RTI40 in BAL fluid is associated with the extent of morphological injury (necrosis) to alveolar epithelial cells in other models of acute lung injury (22-24). It is possible that the increased protein content of BAL fluid may represent increased serum flux into the air spaces secondary to necrosis of type I cells (22). Our data therefore suggest that the additional S. aureus burden in DU5883-infected rats increased the severity of alveolar wall injury in comparison to 8325-4- and DU5883 (pFnBPA)-inoculated lungs at 24 h postinfection. Our data demonstrate that the absence of fibronectin-binding proteins promotes alveolar wall injury in a rat model of S. aureus pneumonia.
Many studies demonstrate that highly encapsulated forms of bacterial pathogens, including S. aureus, are more virulent than the weakly encapsulated forms (5, 35, 36). This difference in virulence is thought to be due to the fact that encapsulated forms are more resistant to internalization by both professional (e.g., neutrophils) and nonprofessional (e.g., epithelial cells) phagocytes in vivo (5, 35, 36). We have not determined why strain DU5883 survives better in lung tissue than 8325-4 and DU5883(pFnBPA4). Our data demonstrate that the number of neutrophils recovered in BAL fluid at 24 h postinfection is not significantly different among the three strains. Moreover, neutrophil recruitment to lung tissue, as measured by myeloperoxidase activity, was not significantly different between 8325-4- and DU5883-infected lungs (unpublished observations).
Soluble fibronectin, which is present in BAL fluid, facilitates neutrophil phagocytosis and killing of S. aureus in in vitro assays (13, 30, 41). The fibronectin-binding protein-deficient S. aureus strain may be less efficiently cleared by neutrophils in comparison with fibronectin-binding protein-expressing strains because it does not bind fibronectin. Alternatively, fibronectin bound to fibronectin-binding protein-expressing strains of S. aureus may enhance alveolar macrophage activation in comparison with the knockout mutant (39). We are currently investigating these two possibilities.
The exact the role of fibronectin-binding proteins in S. aureus virulence is unclear. On one hand, all of the clinical strains of S. aureus tested so far contain one or both fibronectin-binding protein genes (27). No S. aureus strains have been identified which do not contain either of the fibronectin-binding protein genes (27). On the other hand, data from experimental animal studies demonstrate that the expression of fibronectin-binding proteins is not important for S. aureus virulence and may in fact decrease the extent of virulence (8, 15; this study). Interestingly, adhesion of clinical strains of S. aureus to fibronectin-coated slides is variable and does not correlate with the number of fibronectin-binding protein genes (27). Some methicillin-resistant S. aureus strains are also characterized by their low adherence to fibronectin-coated slides (35). Low adherence of these methicillin-resistant S. aureus strains is associated with the expression of a cell surface protein called Pls (plasmin sensitive) (33). It is possible that the antivirulence effects of fibronectin-binding proteins may be masked or modified during infection by the expression of antiadhesion proteins such as Pls.
Integrin antagonists have been proposed as possible new therapeutic agents to aid in the elimination of gram-positive organisms by preventing fibronectin-binding protein-mediated internalization into host cells (6). Our study suggests that such drugs are unlikely to be useful in the treatment of S. aureus-induced pneumonia. While we were able to detect S. aureus within neutrophils at 24 h postinfection in rats inoculated with strain 8325-4, we were not able to detect any S. aureus internalized into alveolar epithelial cells. Our data suggest that S. aureus internalization into alveolar epithelial cells by a fibronectin-binding protein-mediated mechanism is unlikely to be a virulence mechanism in our rat model of pneumonia.
In summary, despite the fact that fibronectin-binding proteins are widely expressed by S. aureus, our data suggest that they reduce S. aureus virulence in a rat model of pneumonia. Our data raise the possibility that anti-fibronectin-binding proteins, such as Pls, may contribute to virulence by preventing S. aureus internalization by both professional and nonprofessional phagocytes.
Acknowledgments
The antibodies against RTI40 and RTII70 were a kind gift from L. G. Dobbs, University of California, San Francisco. SV40-AT2 cells were a generous gift of M. C. Williams, University of Boston. We also thank James Pryde and Joseph Gray for comments on the manuscript.
We acknowledge the Scottish Hospital Endowment Fund for supporting this research (SHERT RG65/99) and a Faculty of Medicine Scholarship (M. C. McElroy).
Editor: B. B. Finlay
REFERENCES
- 1.Ahmed, S., S. Meghji, R. J. Williams, B. Henderson, J. H. Brock, and S. P. Nair. 2001. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect. Immun. 69:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, R. A., K. R. Matthews, E. Cifrian, A. J. Guidry, and S. P. Oliver. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 79:1021-1026. [DOI] [PubMed] [Google Scholar]
- 3.Boylan, G. M., J. G. Pryde, L. G. Dobbs, and M. C. McElroy. 2001. Identification of a novel antigen on the apical surface of rat alveolar epithelial type II and Clara cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L1318-1326. [DOI] [PubMed] [Google Scholar]
- 4.Clement, A., M. P. Steele, J. S. Brody, and N. Riedel. 1991. SV40T-immortalized lung alveolar epithelial cells display posttranscriptional regulation of proliferation-related genes. Exp. Cell Res. 196:198-205. [DOI] [PubMed] [Google Scholar]
- 5.Cortes, G., D. Alvarez, C. Saus, and S. Alberti. 2002. Role of lung epithelial cells in defense against Klebsiella pneumoniae pneumonia. Infect. Immun. 70:1075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cue, D., S. O. Southern, P. J. Southern, J. Prabhakar, W. Lorelli, J. M. Smallheer, S. A. Mousa, and P. P. Cleary. 2000. A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin alpha 5 beta 1-fibronectin-M1 protein complexes. Proc. Natl. Acad. Sci. USA 97:2858-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damjanovich, L., S. M. Albelda, S. A. Mette, and C. A. Buck. 1992. Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am. J. Respir. Cell Mol. Biol. 6:197-206. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche, R. O., G. C. Landon, J. M. Patti, L. L. Nguyen, R. C. Fernau, D. McDevitt, C. Greene, T. Foster, and M. Klima. 1997. Role of Staphylococcus aureus surface adhesins in orthopaedic device infections: are results model-dependent? J. Med. Microbiol. 46:75-79. [DOI] [PubMed] [Google Scholar]
- 9.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-132. [DOI] [PubMed] [Google Scholar]
- 10.Dobbs, L. G., M. C. Williams, and R. Gonzalez. 1988. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim. Biophys. Acta 970:146-156. [DOI] [PubMed] [Google Scholar]
- 11.Dobbs, L. G., M. S. Pian, M. Maglio, S. Dumars, and L. Allen. 1997. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am. J. Physiol. 273:L347-354. [DOI] [PubMed] [Google Scholar]
- 12.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eklund, A., G. Tornling, E. Blaschke, and T. Curstedt. 1991. Extracellular matrix components in bronchoalveolar lavage fluid in quartz exposed rats. Br. J. Ind. Med. 48:776-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 15.Flock, J. I., S. A. Hienz, A. Heimdahl, and T. Schennings. 1996. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect. Immun. 64:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler, T., E. R. Wann, D. Joh, S. Johansson, T. J. Foster, and M. Hook. 2000. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur. J. Cell Biol. 79:672-679. [DOI] [PubMed] [Google Scholar]
- 17.Greene, C., D. McDevitt, P. Francois, P. E. Vaudaux, D. P. Lew, and T. J. Foster. 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 17:1143-1152. [DOI] [PubMed] [Google Scholar]
- 18.Jevon, M., C. Guo, B. Ma, N. Mordan, S. P. Nair, M. Harris, B. Henderson, G. Bentley, and S. Meghji. 1999. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect. Immun. 67:2677-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Hook. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 20.Lowy, D. F. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341-342. [DOI] [PubMed] [Google Scholar]
- 21.Lowy, F. D., J. Fant, L. L. Higgins, S. K. Ogawa, and V. B. Hatcher. 1988. Staphylococcus aureus-human endothelial cell interactions. J. Ultrastruct. Mol. Struct. Res. 98:137-146. [DOI] [PubMed] [Google Scholar]
- 22.McElroy, M. C., J. F. Pittet, S. Hashimoto, L. Allen, J. P. Wiener-Kronish, and L. G. Dobbs. 1995. A type I cell-specific protein is a biochemical marker of epithelial injury in a rat model of pneumonia. Am. J. Physiol. 268:L181-186. [DOI] [PubMed] [Google Scholar]
- 23.McElroy, M. C., J. F. Pittet, L. Allen, J. P. Wiener-Kronish, and L. G. Dobbs. 1997. Biochemical detection of type I cell damage after nitrogen dioxide-induced lung injury in rats. Am. J. Physiol. 273:L1228-1234. [DOI] [PubMed] [Google Scholar]
- 24.McElroy, M. C., H. R. Harty, G. E. Hosford, G. M. Boylan, J. F. Pittet, and T. J. Foster. 1999. Alpha-toxin damages the air-blood barrier of the lung in a rat model of Staphylococcus aureus-induced pneumonia. Infect. Immun. 67:5541-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaut, P., C. Planes, B. Escoubet, A. Clement, C. Amiel, and C. Clerici. 1996. Rat lung alveolar type II cell line maintains sodium transport characteristics of primary culture. J. Cell. Physiol. 169:78-86. [DOI] [PubMed] [Google Scholar]
- 26.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 27.Peacock, S. J., N. P. J. Day, M. G. Thomas, A. R. Berendt, and T. J. Foster. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23-31. [DOI] [PubMed] [Google Scholar]
- 28.Pottratz, S. T., and A. L. Weir. 1995. Attachment of Pneumocystis carinii to primary cultures of rat alveolar epithelial cells. Exp. Cell Res. 221:357-362. [DOI] [PubMed] [Google Scholar]
- 29.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p. 55-81. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human diseases. Churchill Livingstone, New York, N.Y.
- 30.Rennard, S. I., and R. G. Crystal. 1982. Fibronectin in human bronchopulmonary lavage fluid. Elevation in patients with interstitial lung disease. J. Clin. Investig. 69:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rishi, A. K., M. Joyce-Brady, J. Fisher, L. G. Dobbs, J. Floros, J. VanderSpek, J. S. Brody, and M. C. Williams. 1995. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev. Biol. 167:294-306. [DOI] [PubMed] [Google Scholar]
- 32.Rosenkrans, W. A., Jr., J. T. Albright, R. E. Hausman, and D. P. Penney. 1983. Ultrastructural immunocytochemical localization of fibronectin in the developing rat lung. Cell Tissue Res. 234:165-177. [DOI] [PubMed] [Google Scholar]
- 33.Savolainen, K., L. Paulin, B. Westerlund-Wikstrom, T. J. Foster, T. K. Korhonen, and P. Kuusela. 2001. Expression of pls, a gene closely associated with the mecA gene of methicillin-resistant Staphylococcus aureus, prevents bacterial adhesion in vitro. Infect. Immun. 69:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz, F. J., A. C. Fluit, M. Gondolf, R. Beyrau, E. Lindenlauf, J. Verhoef, H. P. Heinz, and M. E. Jones. 1999. The prevalence of aminoglycoside resistance and corresponding resistance genes in clinical isolates of staphylococci from 19 European hospitals. J. Antimicrob. Chemother. 43:253-259. [PubMed] [Google Scholar]
- 35.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infections. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thakker, M., J. S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzagloff, H., and R. Novick. 1977. Geometry of cell division in Staphylococcus aureus. J. Bacteriol. 129:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uphoff, C. C., S. M. Gignac, and D. G. Drexler. 1992. Mycoplasma contamination in human leukemia cell lines. J. Immunol. Methods 149:43-53. [DOI] [PubMed] [Google Scholar]
- 39.Vassallo, R., T, J. Kottom, J. E. Standing, and A. H. Limper. 2001. Vitronectin and fibronectin function as glucan binding proteins augmenting macrophage responses to Pneumocystis carinii. Am. J. Respir. Cell Mol. Biol. 25:203-211. [DOI] [PubMed] [Google Scholar]
- 40.Wolfensohn, S., and M. Lloyd. 1998. Small laboratory animals, p.169-217. In Handbook of laboratory animal management and Welfare, 2nd ed. Blackwell Science, London, England.
- 41.Yang, K. D., N. H. Augustine, L. A. Gonzalez, J. F. Bohnsack, and H. R. Hill. 1988. Effects of fibronectin on the interaction of polymorphonuclear leukocytes with unopsonized and antibody-opsonized bacteria. J. Infect. Dis. 158:823-830. [DOI] [PubMed] [Google Scholar]