Abstract
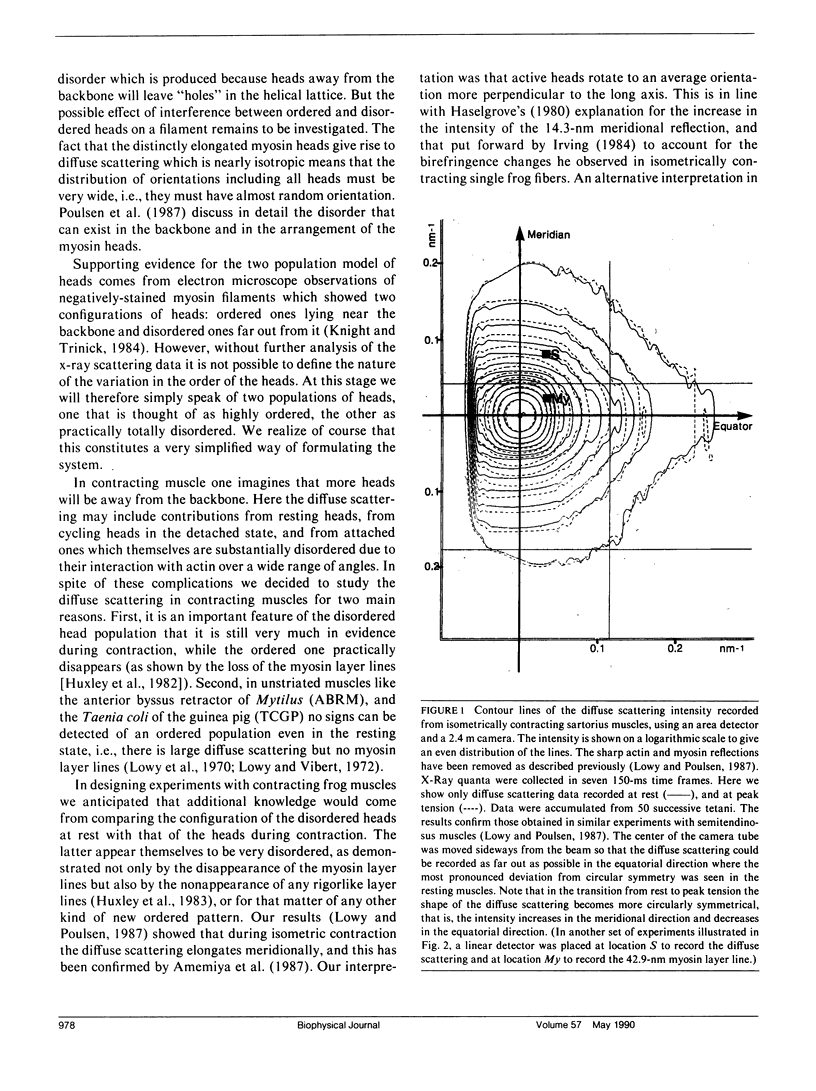
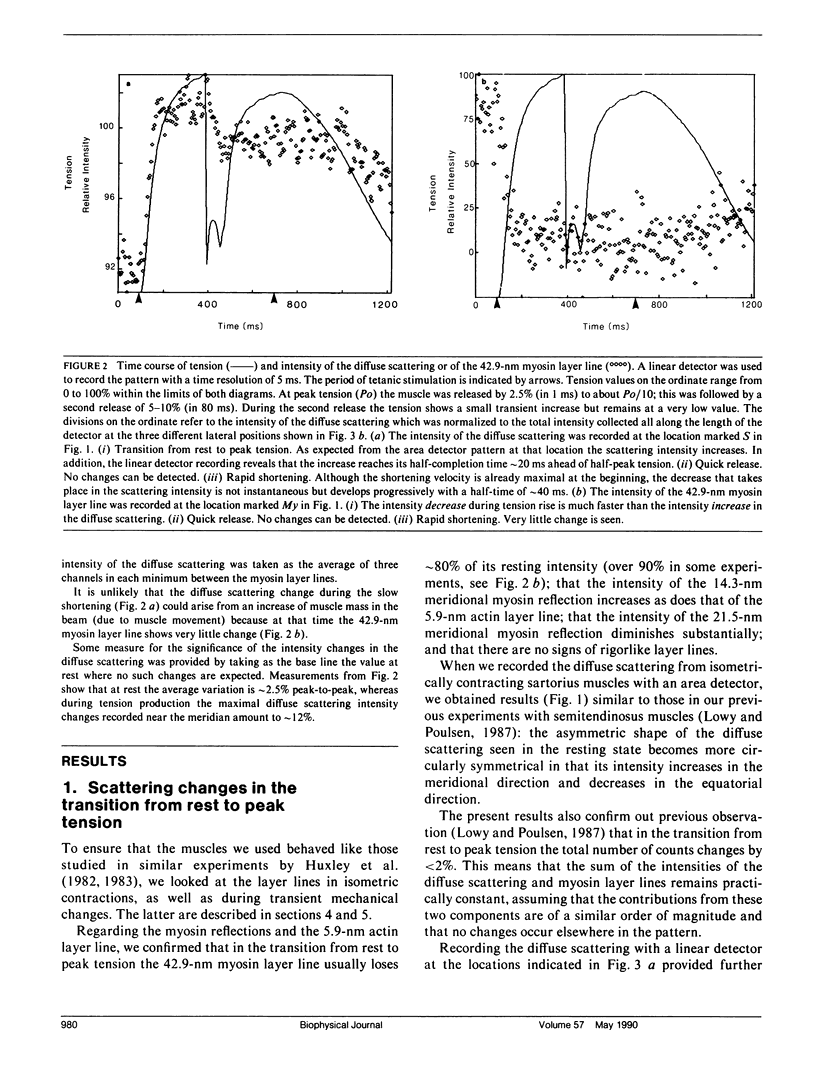
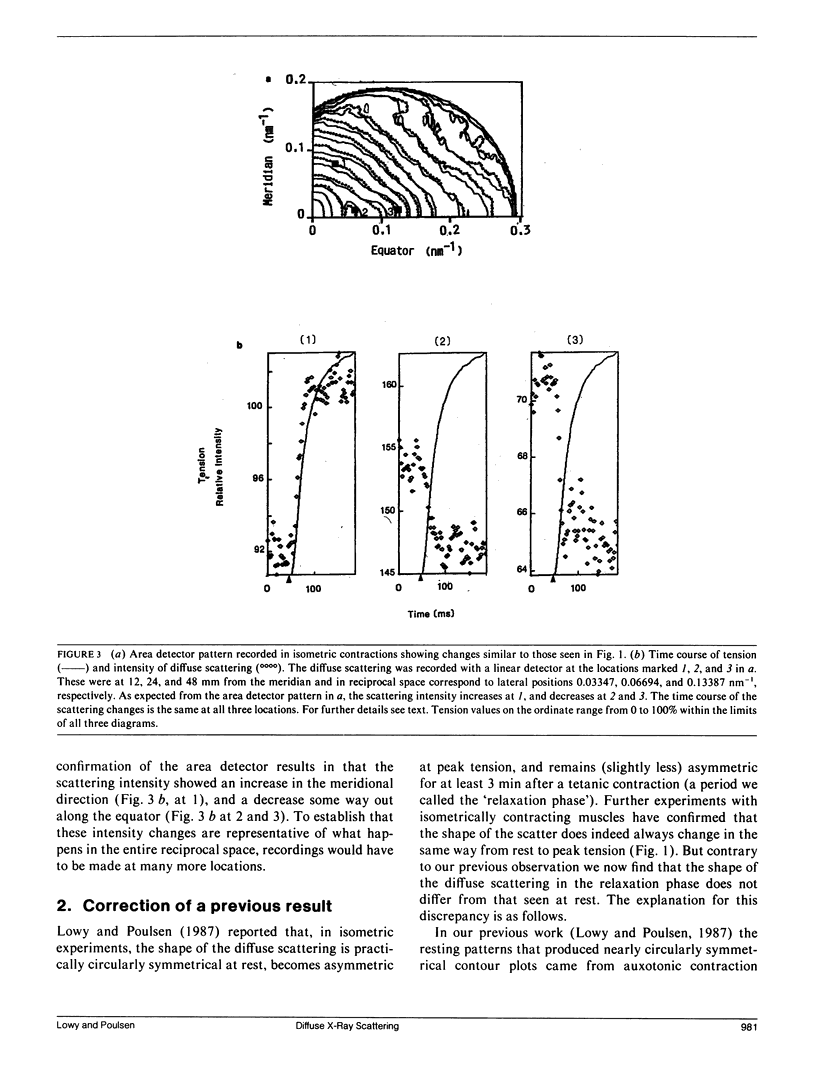
Using x-rays from synchrotron radiation, we studied diffuse scattering, sometimes together with the myosin layer lines. With an area detector, sartorius muscles and a time resolution of 150 ms, earlier results from semitendinosus muscles contracting isometrically at 6 degrees C (Lowy, J., and F. R. Poulsen. 1987. J. Mol. Biol. 194:595-600) were confirmed and extended. Evidence from intensity changes both in the diffuse scattering and in the myosin layer lines showed that the majority of the heads become disordered at peak tetanic tension. With a linear detector and a time resolution of 5 ms, it was found that during tension rise the intensity increase of the diffuse scattering (which amounted maximally to 12% recorded near the meridian) runs approximately 20 ms ahead of the mechanical change, comparing half-completion times. This suggests that an appreciable number of heads change orientation before peak tension is reached. In quick release experiments the diffuse scattering intensity showed very little change. Recorded near the meridian during rapid shortening, however, it decreased progressively with a half-time of approximately 40 ms. This change amounted to approximately 35% of that observed during the initial tension rise. We interpret this to indicate that during rapid shortening a certain number of heads assume an orientation characteristic of the relaxed state. Viewed in the context of the behavior of the first myosin layer line and the (1, 1) equatorial reflection in similar experiments (Huxley, H. E., M. Kress, A. R. Faruqi, and R. M. Simmons. 1988. Molecular Mechanism of Muscle Contraction), the present results provide further support for the view that the diffuse scattering is mostly due to disordered myosin heads; whilst ordered heads produce the myosin layer lines (Poulsen, F. R., and J. Lowy.1983. Nature lLond.l. 303:146-152).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemiya Y., Wakabayashi K., Tanaka H., Ueno Y., Miyahara J. Laser-stimulated luminescence used to measure x-ray diffraction of a contracting striated muscle. Science. 1987 Jul 10;237(4811):164–168. doi: 10.1126/science.3496662. [DOI] [PubMed] [Google Scholar]
- Castellani L., Vibert P., Cohen C. Structure of myosin/paramyosin filaments from a molluscan smooth muscle. J Mol Biol. 1983 Jul 15;167(4):853–872. doi: 10.1016/s0022-2836(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Harford J., Squire J. "Crystalline" myosin cross-bridge array in relaxed bony fish muscle. Low-angle x-ray diffraction from plaice fin muscle and its interpretation. Biophys J. 1986 Jul;50(1):145–155. doi: 10.1016/S0006-3495(86)83447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C. A model of myosin crossbridge structure consistent with the low-angle x-ray diffraction pattern of vertebrate muscle. J Muscle Res Cell Motil. 1980 Jun;1(2):177–191. doi: 10.1007/BF00711798. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Kress M., Bordas J., Koch M. H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982 Jul 15;158(4):637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications. J Mol Biol. 1983 Sep 15;169(2):469–506. doi: 10.1016/s0022-2836(83)80062-x. [DOI] [PubMed] [Google Scholar]
- Knight P., Trinick J. Structure of the myosin projections on native thick filaments from vertebrate skeletal muscle. J Mol Biol. 1984 Aug 15;177(3):461–482. doi: 10.1016/0022-2836(84)90295-x. [DOI] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R. Time-resolved X-ray diffraction studies of the structural behaviour of myosin heads in a living contracting unstriated muscle. Nature. 1982 Sep 23;299(5881):308–312. doi: 10.1038/299308a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R., Vibert P. J. Myosin filaments in vertebrate smooth muscle. Nature. 1970 Mar 14;225(5237):1053–1054. doi: 10.1038/2251053a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R. X-ray study of myosin heads in contracting frog skeletal muscle. J Mol Biol. 1987 Apr 20;194(4):595–600. doi: 10.1016/0022-2836(87)90236-1. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Millman B. M. X-ray diffraction patterns from mammalian heart muscle. J Mol Biol. 1974 Feb 5;82(4):527–536. doi: 10.1016/0022-2836(74)90246-0. [DOI] [PubMed] [Google Scholar]
- Poulsen F. R., Lowy J., Cooke P. H., Bartels E. M., Elliott G. F., Hughes R. A. Diffuse X-ray scatter from myosin heads in oriented synthetic filaments. Biophys J. 1987 Jun;51(6):959–967. doi: 10.1016/S0006-3495(87)83423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen F. R., Lowy J. Small-angle X-ray scattering from myosin heads in relaxed and rigor frog skeletal muscles. Nature. 1983 May 12;303(5913):146–152. doi: 10.1038/303146a0. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



