Abstract
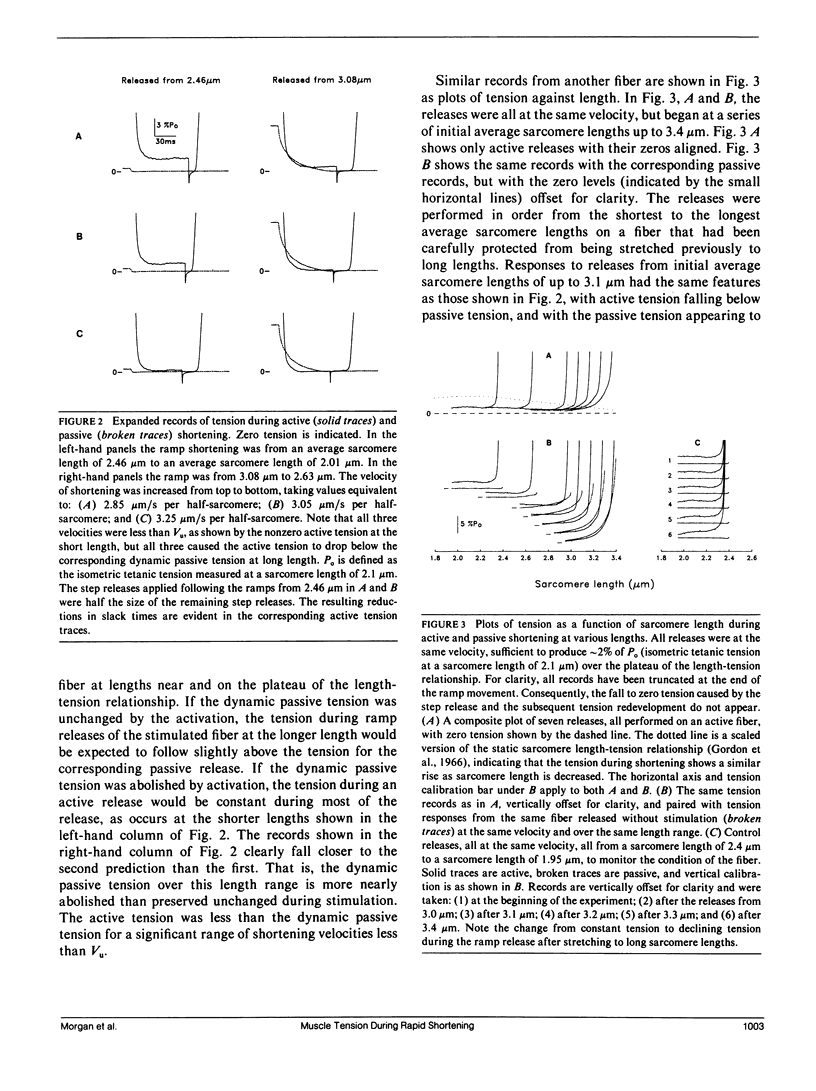
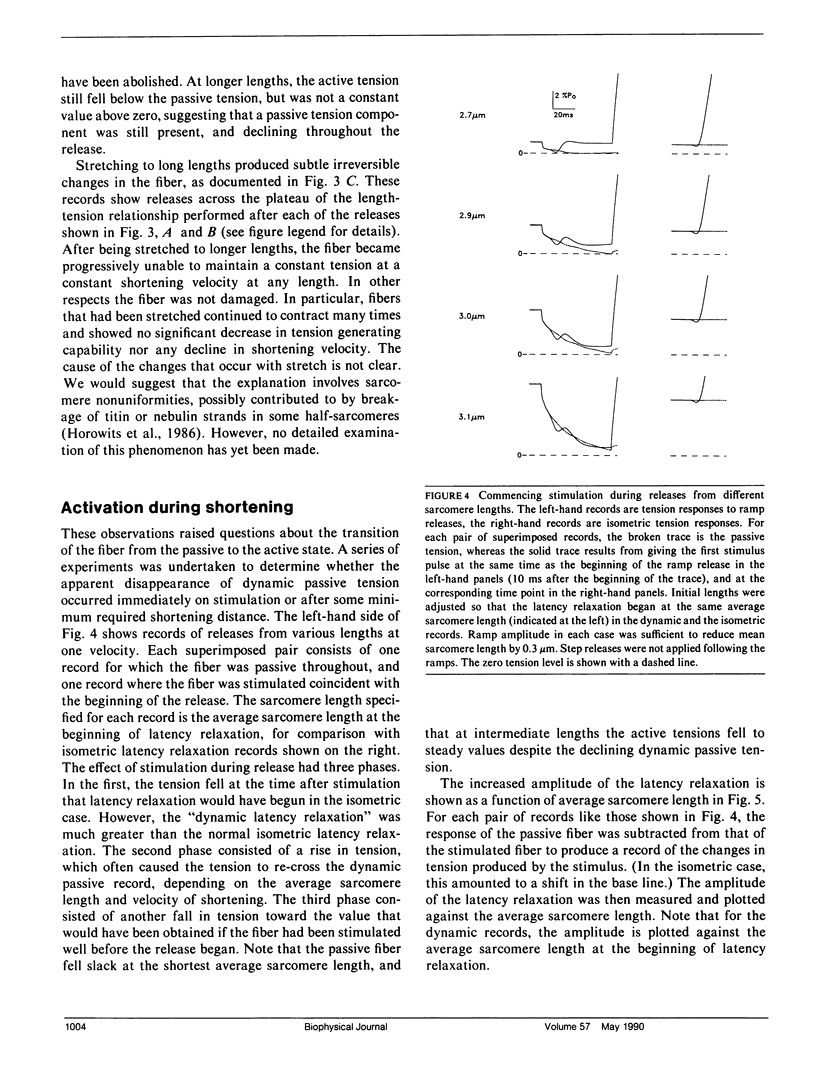
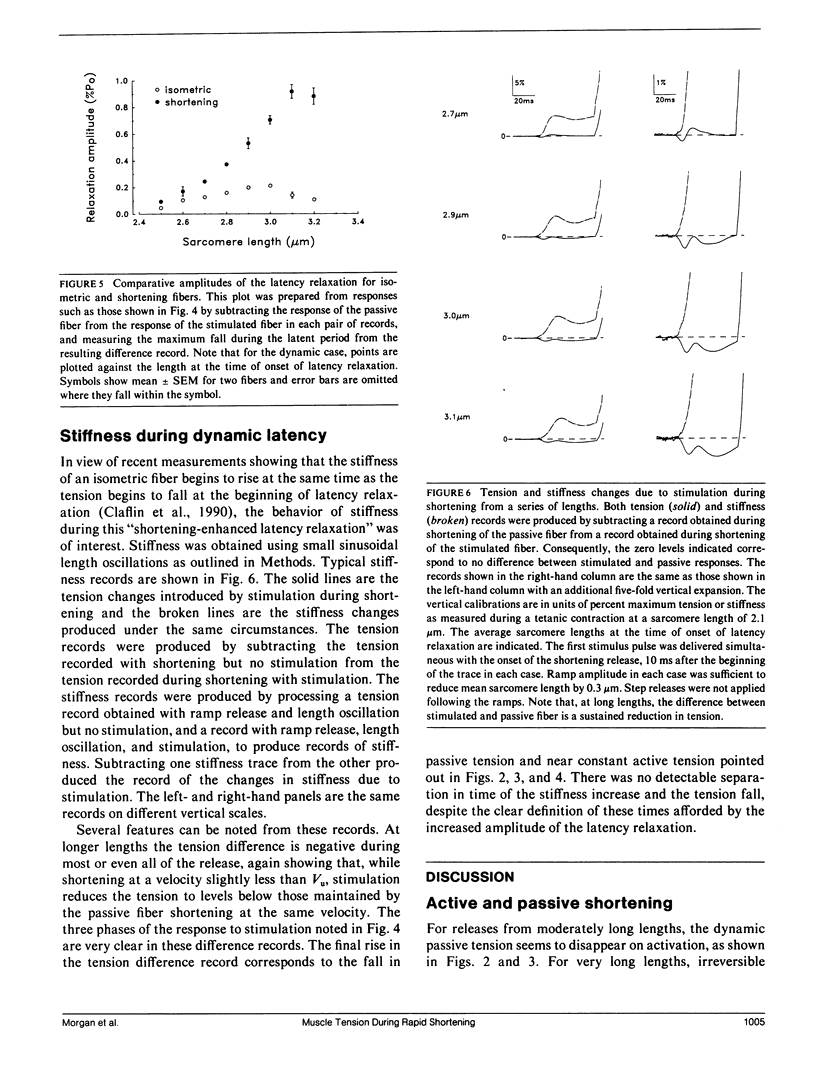
Experiments were undertaken to determine the contribution of passive tension to total tension during rapid shortening in a stimulated muscle fiber. Results were obtained by applying shortening movements at constant velocities slightly less than Vu (the velocity of unloaded shortening) to intact twitch fibers isolated from the frog (Rana temporaria). The tension maintained by unstimulated fibers during such shortening movements ("dynamic passive tension") from moderately long lengths was greater than zero but much less than the passive tension measured under static conditions ("static passive tension") at the same lengths. Fibers maximally activated by electrical stimulation and then shortened at the same velocity over the same range of average sarcomere lengths maintained tension that was greater than zero but less than the dynamic passive tension. For average sarcomere lengths up to approximately 3.1 microns, the dynamic passive tension appeared to be substantially abolished by activation. The onset of the apparent disappearance of dynamic passive tension was studied by initiating the stimulation and the shortening movement simultaneously. The resulting tension response exhibited a latency relaxation that was increased in amplitude compared with the isometric case, followed by a brief tension rise, giving way to a steady tension level equal to that expected if stimulation had been initiated well before the release. These changes are qualitatively explained in terms of the establishment of a steady state distribution of deformations of attached cross-bridges.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutsaert D. L., Claes V. A., Sonnenblick E. H. Velocity of shortening of unloaded heart muscle and the length-tension relation. Circ Res. 1971 Jul;29(1):63–75. doi: 10.1161/01.res.29.1.63. [DOI] [PubMed] [Google Scholar]
- Claflin D. R., Morgan D. L., Julian F. J. Earliest mechanical evidence of cross-bridge activity after stimulation of single skeletal muscle fibers. Biophys J. 1990 Mar;57(3):425–432. doi: 10.1016/S0006-3495(90)82559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin D. R., Morgan D. L., Julian F. J. Effects of passive tension on unloaded shortening speed of frog single muscle fibers. Biophys J. 1989 Nov;56(5):967–977. doi: 10.1016/S0006-3495(89)82742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Haugen P., Sten-Knudsen O. Sarcomere lengthening and tension drop in the latent period of isolated frog skeletal muscle fibers. J Gen Physiol. 1976 Sep;68(3):247–265. doi: 10.1085/jgp.68.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R., Kempner E. S., Bisher M. E., Podolsky R. J. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986 Sep 11;323(6084):160–164. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Intersarcomere dynamics during fixed-end tetanic contractions of frog muscle fibres. J Physiol. 1979 Aug;293:365–378. doi: 10.1113/jphysiol.1979.sp012894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woledge R. C., Curtin N. A., Homsher E. Energetic aspects of muscle contraction. Monogr Physiol Soc. 1985;41:1–357. [PubMed] [Google Scholar]


