Abstract
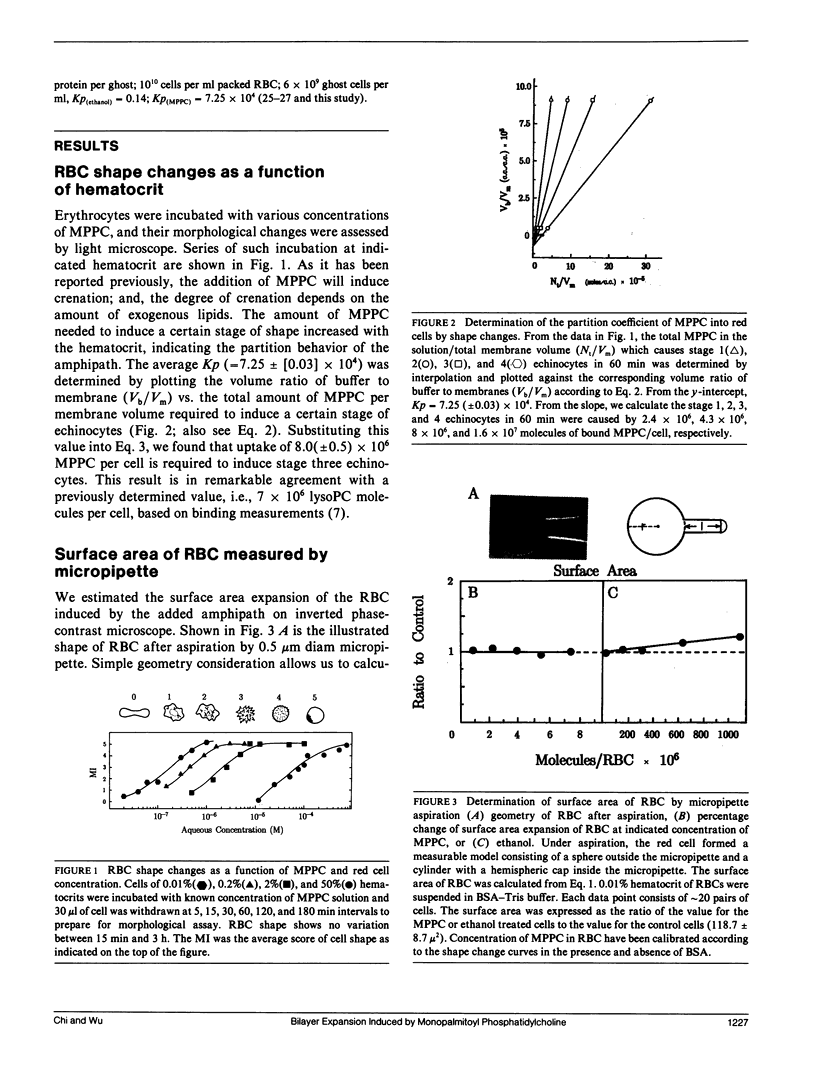
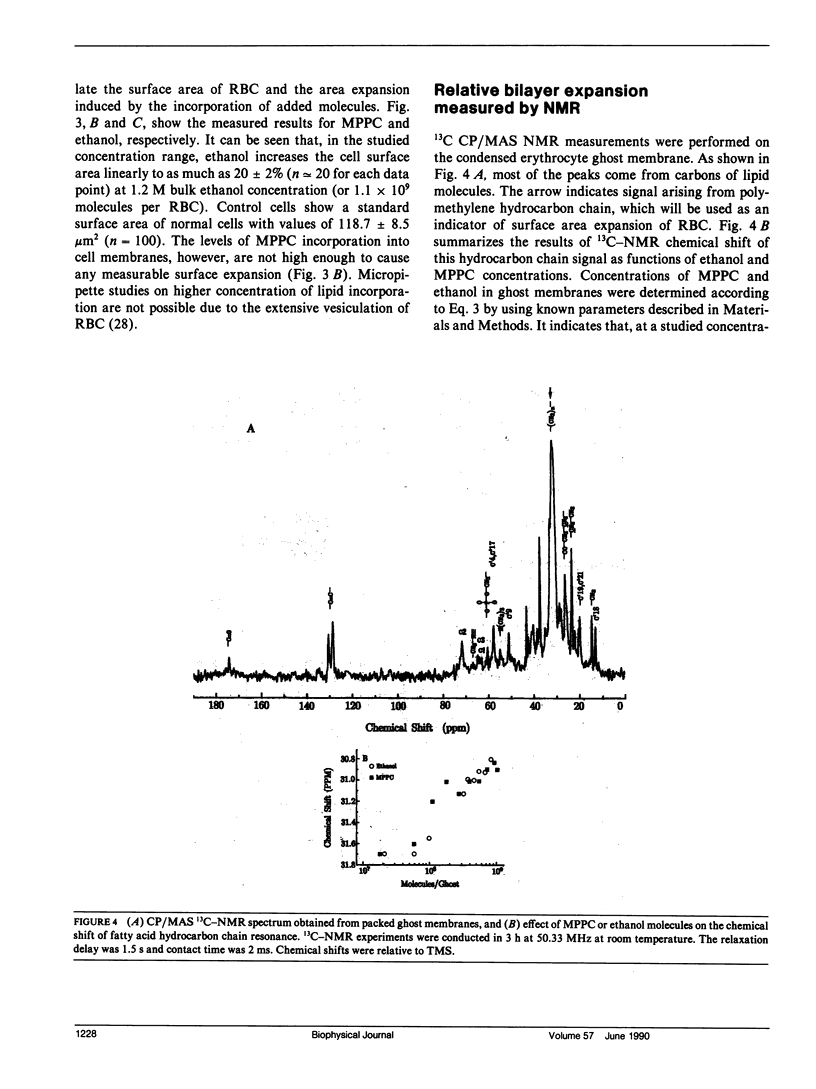
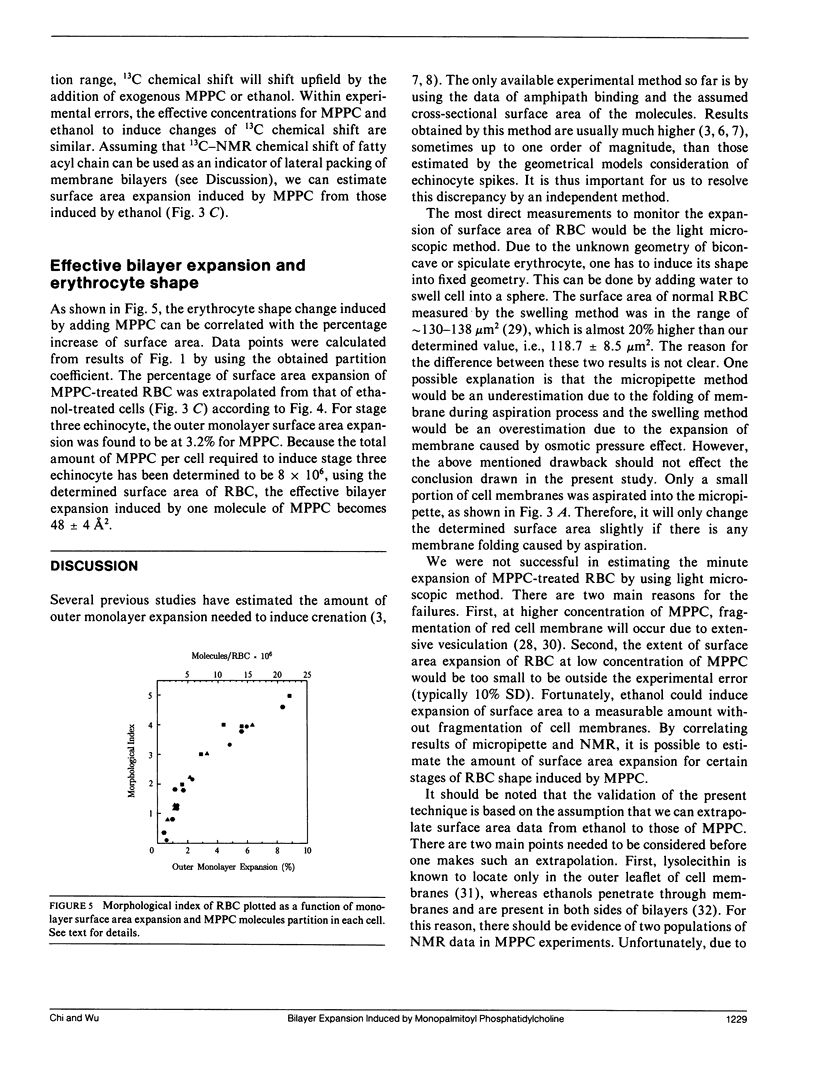
When human erythrocytes are treated with exogenous monopalmitoyl phosphatidylcholine (MPPC), the normal biconcave disk shape red blood cells (RBC) become spiculate echinocytes. The present study examines the quantitative aspect of the relationship between effective bilayer expansion and erythrocyte shape change by a newly developed method. This method is based on the combination of direct surface area measurement of micropipette and relative bilayer expansion measurement of 13C crosspolarization/magic angle spinning nuclear magnetic resonance (NMR). Assuming that 13C NMR chemical shift of fatty acyl chain can be used as an indicator of lateral packing of membrane bilayers, it is possible for us to estimate the surface area expansion of red cell membrane induced by MPPC from that induced by ethanol. Partitions of lipid molecules into cell membrane were determined by studies of shape change potency as a function of MPPC and red cell concentration. It is found that 8(+/- 0.5) x 10(6) molecules of MPPC per cell will effectively induce stage three echinocytes and yield 3.2(+/- 0.2)% expansion of outer monolayer surface area. Surface area of normal cells determined by direct measurements from fixed geometry of red cells aspirated by micropipette was 118.7 +/- 8.5 microns2. The effective cross-sectional area of MPPC molecules in the cell membrane therefore was determined to be 48(+/- 4) A2, which is in agreement with those determined by x-ray from model membranes and crystals of lysophospholipids. We concluded that surface area expansion of RBC can be explained by a simple consideration of cross-sectional area of added molecules and that erythrocyte shape changes correspond quantitatively to the incorporated lipid molecules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Noguchi C. T., Schechter A. N., Torchia D. A. Proton-enhanced 13C NMR of normal human erythrocytes: characterization of motionally restricted molecules. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1161–1168. doi: 10.1016/0006-291x(82)91234-7. [DOI] [PubMed] [Google Scholar]
- Batchelor J. G., Prestegard J. H., Cushley R. J., Lipsky S. R. Conformational analysis of lecithin in vesicles by 13 C NMR. Biochem Biophys Res Commun. 1972 Jul 11;48(1):70–75. doi: 10.1016/0006-291x(72)90345-2. [DOI] [PubMed] [Google Scholar]
- Bergmann W. L., Dressler V., Haest C. W., Deuticke B. Reorientation rates and asymmetry of distribution of lysophospholipids between the inner and outer leaflet of the erythrocyte membrane. Biochim Biophys Acta. 1984 May 30;772(3):328–336. doi: 10.1016/0005-2736(84)90150-0. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Tilly R. H., Senden J. M., Comfurius P., Zwaal R. F. Exposure of endogenous phosphatidylserine at the outer surface of stimulated platelets is reversed by restoration of aminophospholipid translocase activity. Biochemistry. 1989 Mar 21;28(6):2382–2387. doi: 10.1021/bi00432a007. [DOI] [PubMed] [Google Scholar]
- Bierbaum T. J., Bouma S. R., Huestis W. H. A mechanism of erythrocyte lysis by lysophosphatidylcholine. Biochim Biophys Acta. 1979 Jul 19;555(1):102–110. doi: 10.1016/0005-2736(79)90075-0. [DOI] [PubMed] [Google Scholar]
- Chien S., Sung K. L., Skalak R., Usami S., Tözeren A. Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophys J. 1978 Nov;24(2):463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J., Schroit A. J. Transbilayer movement of phosphatidylserine in erythrocytes: inhibition of transport and preferential labeling of a 31,000-dalton protein by sulfhydryl reactive reagents. Biochemistry. 1988 Feb 9;27(3):848–851. doi: 10.1021/bi00403a002. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Daleke D. L., Huestis W. H. Incorporation and translocation of aminophospholipids in human erythrocytes. Biochemistry. 1985 Sep 24;24(20):5406–5416. doi: 10.1021/bi00341a019. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Huestis W. H. Phosphoinositide metabolism and the morphology of human erythrocytes. J Cell Biol. 1984 Jun;98(6):1992–1998. doi: 10.1083/jcb.98.6.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Lee K. J., Huestis W. H. Membrane bilayer balance and erythrocyte shape: a quantitative assessment. Biochemistry. 1985 Jun 4;24(12):2849–2857. doi: 10.1021/bi00333a006. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J., Ekholm J. E. The preparation of red cell ghosts (membranes). Methods Enzymol. 1974;31:168–172. doi: 10.1016/0076-6879(74)31018-x. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Conformation of phospholipids. Crystal structure of a lysophosphatidylcholine analogue. J Mol Biol. 1980 Mar 5;137(3):249–264. doi: 10.1016/0022-2836(80)90315-0. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Huang C. H. X-ray diffraction evidence for fully interdigitated bilayers of 1-stearoyllysophosphatidylcholine. Biochemistry. 1986 Mar 25;25(6):1330–1335. doi: 10.1021/bi00354a021. [DOI] [PubMed] [Google Scholar]
- Isomaa B., Hägerstrand H., Paatero G. Shape transformations induced by amphiphiles in erythrocytes. Biochim Biophys Acta. 1987 May 12;899(1):93–103. doi: 10.1016/0005-2736(87)90243-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange Y., Gough A., Steck T. L. Role of the bilayer in the shape of the isolated erythrocyte membrane. J Membr Biol. 1982;69(2):113–123. doi: 10.1007/BF01872271. [DOI] [PubMed] [Google Scholar]
- Lange Y., Slayton J. M. Interaction of cholesterol and lysophosphatidylcholine in determining red cell shape. J Lipid Res. 1982 Nov;23(8):1121–1127. [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Bayne H., Markus J. Relationship of hyperthermia-induced hemolysis of human erythrocytes to the thermal denaturation of membrane proteins. Biochim Biophys Acta. 1989 Apr 14;980(2):191–201. doi: 10.1016/0005-2736(89)90399-4. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Lange Y., Weinstein R. S., Steck T. L. Interaction of chlorpromazine with the human erythrocyte membrane. J Biol Chem. 1984 Jul 25;259(14):9225–9234. [PubMed] [Google Scholar]
- Maciel G. E. High-resolution nuclear magnetic resonance of solids. Science. 1984 Oct 19;226(4672):282–288. doi: 10.1126/science.226.4672.282. [DOI] [PubMed] [Google Scholar]
- Matayoshi E. D. Distribution of shape-changing compounds across the red cell membrane. Biochemistry. 1980 Jul 22;19(15):3414–3422. doi: 10.1021/bi00556a002. [DOI] [PubMed] [Google Scholar]
- McLawhon R. W., Marikovsky Y., Thomas N. J., Weinstein R. S. Ethanol-induced alterations in human erythrocyte shape and surface properties: modulatory role of prostaglandin E1. J Membr Biol. 1987;99(1):73–78. doi: 10.1007/BF01870623. [DOI] [PubMed] [Google Scholar]
- Middlekoop E., Van der Hoek E. E., Bevers E. M., Comfurius P., Slotboom A. J., Op den Kamp J. A., Lubin B. H., Zwaal R. F., Roelofsen B. Involvement of ATP-dependent aminophospholipid translocation in maintaining phospholipid asymmetry in diamide-treated human erythrocytes. Biochim Biophys Acta. 1989 May 19;981(1):151–160. doi: 10.1016/0005-2736(89)90093-x. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Bowers J. L., Forbes J. High-resolution proton and carbon-13 NMR of membranes: why sonicate? Biochemistry. 1987 Nov 3;26(22):6919–6923. doi: 10.1021/bi00396a009. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Pangborn W. A., Purdon A. D., Tinker D. O. Lysolecithin and cholesterol interact stoichiometrically forming bimolecular lamellar structures in the presence of excess water, of lysolecithin or cholesterol. Can J Biochem. 1975 Feb;53(2):189–195. doi: 10.1139/o75-027. [DOI] [PubMed] [Google Scholar]
- Seeman P., Kwant W. O., Sauks T. Membrane expansion of erythrocyte ghosts by tranquilizers and anesthetics. Biochim Biophys Acta. 1969;183(3):499–511. doi: 10.1016/0005-2736(69)90164-3. [DOI] [PubMed] [Google Scholar]
- Seeman P., Roth S., Schneider H. The membrane concentrations of alcohol anesthetics. Biochim Biophys Acta. 1971 Feb 2;225(2):171–184. doi: 10.1016/0005-2736(71)90210-0. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret M., Zachowski A., Hermann A., Devaux P. F. Asymmetric lipid fluidity in human erythrocyte membrane: new spin-label evidence. Biochemistry. 1984 Sep 11;23(19):4271–4275. doi: 10.1021/bi00314a002. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Painter R. G., Singer S. J. Biological membranes as bilayer couples. III. Compensatory shape changes induced in membranes. J Cell Biol. 1976 Jul;70(1):193–203. doi: 10.1083/jcb.70.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sune A., Bette-Bobillo P., Bienvenüe A., Fellmann P., Devaux P. F. Selective outside-inside translocation of aminophospholipids in human platelets. Biochemistry. 1987 Jun 2;26(11):2972–2978. doi: 10.1021/bi00385a003. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Williamson P., Antia R., Schlegel R. A. Maintenance of membrane phospholipid asymmetry. Lipid-cytoskeletal interactions or lipid pump? FEBS Lett. 1987 Jul 27;219(2):316–320. doi: 10.1016/0014-5793(87)80243-0. [DOI] [PubMed] [Google Scholar]



