Abstract
Gram-negative bacterial proteins which are exported from the cytosol to the external environment by the type V secretion system are also known as autotransporters. Once translocated to the periplasmic compartment by the sec-dependent general secretory pathway, their C-terminal domain forms a pore through which the N-terminal domain travels to the outer membrane without the need of other accessory proteins. MisL (protein of membrane insertion and secretion) is a protein of unknown function located in the pathogenicity island SPI-3 of Salmonella enterica and classified as an autotransporter due to its high homology to Escherichia coli AIDA-I. In the present work, the MisL C-terminal translocator domain was used to display the immunodominant B-cell epitope of the circumsporozoite protein (CSP) from Plasmodium falciparum on the surface of Salmonella enterica serovar Typhimurium (serovar Typhimurium SL3261) and serovar Typhi (serovar Typhi CVD 908). The MisL β domain was predicted by alignment with AIDA-I, amplified from serovar Typhimurium SL3261, cloned in a plasmid fused to four repeats of the tetrapeptide NANP behind the Escherichia coli heat-labile enterotoxin B subunit signal peptide to ensure periplasmic traffic, and expressed under the control of the anaerobically inducible nirB promoter. The fusion protein was translocated to the outer membrane of both bacterial strains, although the foreign epitope was displayed more efficiently in serovar Typhimurium SL3261, which elicited a better specific antibody response in BALB/c mice. More importantly, antibodies were able to recognize the native CSP in P. falciparum sporozoites. These results confirm that MisL is indeed an autotransporter and that it can be used to express foreign immunogenic epitopes on the surface of gram-negative bacteria.
Gram-negative bacteria have evolved at least five different systems to translocate proteins from the cytosol to the external environment. The type V secretion system comprises the proteins known as autotransporters (12). The general structure of these proteins consists of three different functional regions. The N-terminal signal sequence is removed after the protein is translocated from the inner membrane to the periplasmic space presumably via the sec system. The C-terminal hydrophobic β domain contains the transporting function, as it folds in antiparallel β strands, forming a transmembrane barrel similar to the bacterial porins. Finally, the N-terminal passenger α domain contains the biological activity and travels through the pore to the external environment. Once translocated to the bacterial surface, these proteins may remain attached to the external membrane, be cleaved by other proteases, or be released to the external milieu by an autocatalytic mechanism (10).
Approximately 40 proteins with autotransporting properties have been recognized, most of which are encoded by single genes located in pathogenicity islands and function as virulence factors involved mainly in adhesion or proteolysis (13). The immunoglobulin A (IgA) protease of Neisseria gonorrhoeae was the first autotransporter described with this function (29), but other proteins containing a consensus serine protease active site (GDSGSG) (9) have been found and grouped in the subfamily of serine protease autotransporters of the family Enterobacteriaceae. Members include SigA from Shigella dysenteriae (1), Pic from Shigella flexneri and enteroaggregative Escherichia coli (11), Sat of uropathogenic E. coli (9), and Tsh from avian-pathogenic E. coli (6). Autotransporters with afimbrial adhesion properties include AIDA-I (adhesin involved in diffuse adherence) of enteropathogenic E. coli (2), TibA of enterotoxigenic E. coli (24), the pertactin precursor from Bordetella pertussis (23), and Hia from Haemophilus influenzae (33). Moreover, autotransporters have been implicated in other virulence mechanisms: for instance, VirG (IcsA) mediates the spreading of Shigella between cells by eliciting polar deposition of actin in the cytoplasm of epithelial cells (34).
Autotransporters have been demonstrated to be feasible tools to display foreign passenger peptides on the bacterial surface because they do not require participation of accessory proteins and are able to translocate a broad range of passenger peptides or proteins, which maintain their antigenicity and biological functions. Therefore, autotransporter-mediated surface display (autodisplay) is especially attractive for the development of live-vector bacterial vaccines. The Neisseria IgA protease β domain has been able to translocate the cholera toxin B (CTB) subunit to the surface of E. coli (19) and to translocate single-chain antibody (scFv) which was able to pass through the outer membrane in an active conformation with its disulfide bonds, in opposition to the notion that only unfolded passenger domains could be translocated (36). AIDA-I from enteropathogenic E. coli has been used to display enzymatically active β-lactamase on the surface of E. coli (22), functional T-cell epitopes of the heat shock protein 60 (Y-hsp60) from Yersinia enterocolitica (20), and the CTB subunit (25). Moreover, CTB can also be released from the cell surface by OmpT-mediated cleavage (25).
Two autotransporters have been identified in Salmonella species by the high degree of homology of their C-terminal domains with AIDA-I. ShdA (protein for efficient and prolonged bacterial persistence by shedding mechanism) is found in domestic fowl-associated serotypes (Salmonella enterica subspecies I) but is absent in reptile-associated serotypes (Salmonella bongori and S. enterica subspecies II to VII) (18). MisL (protein of membrane insertion and secretion) is predicted from an open reading frame identified in the third pathogenicity island (SPI-3) of S. enterica (3), for which a function has not been identified.
In the present work, the MisL β domain was predicted by sequence alignment with AIDA-I (2) and amplified by PCR from S. enterica serovar Typhimurium SL3261. It was cloned under the control of the nirB inducible promoter (28), behind E. coli heat-labile enterotoxin B subunit (LTB) signal peptide, followed by four repeats of the tetrapeptide Asp-Ala-Asp-Pro (NANP), which is the immunodominant B-cell epitope of the circumsporozoite protein (CSP) from Plasmodium falciparum (27). The fusion protein was produced in Salmonella serovar Typhimurium SL3261 and S. enterica serovar Typhi CVD 908, and the passenger epitope was displayed on the surface of both bacterial strains. Immunization of mice with these live vectors elicited antibodies against P. falciparum sporozoites, thus demonstrating that MisL is indeed an autotransporter and that the β domain may be used to display foreign sequences in Salmonella vaccine strains.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used were the following: E. coli DH5α (supE44 Δlac169 φ80lacZΔM15 hsdR17 recA1 endA1 gyrA96 thi-1 relA1), S. enterica serovar Typhimurium SL3261 (aroA::Tn10) (16), and S. enterica serovar Typhi CVD 908 (Ty2 ΔaroC ΔaroD) (17).
Plasmids.
A summary of the plasmids and primers used in this work is presented in Tables 1 and 2. pnirBLTBsp (2,425 bp), derived from pTETnir15, contains a gene encoding the LTB signal sequence (MNKVKFYVLFTALLSSLCAHG) under the control of nirB (unpublished data). pnirBMisL (3,949 bp) contains a gene encoding the LTB signal sequence and the MisL translocator β domain under the control of the nirB promoter (reported in this work). pnirBMisL-NANP (4,005 bp) contains a gene encoding the LTB signal sequence, the (NANP)4 passenger sequence, and the MisL translocator β domain under the control of the nirB promoter (reported in this work). pUC19-CSP (3,886 bp) contains a gene encoding a truncated form of the CSP (amino acids 20 to 381), which produces a cytosolic recombinant protein under the control of the lac promoter.
TABLE 1.
Plasmids
| Name | Size (bp) | Origin | Main component(s) | Promoter | Expected product | NANP epitope
|
|
|---|---|---|---|---|---|---|---|
| Location | Expression | ||||||
| pnirBLTBsp | 2,425 | pTETnir15 | LTB signal sequence (MNKVKFYVLFTALLSSLCAHG) | nirB | Undetectable | ||
| pnirBMisL | 3,949 | pnirBLTBsp | LTB signal sequence and the MisL translocator β domain | nirB | 70 kDa | ||
| pnirBMisL-NANP | 4,005 | pnirBMisL | LTB signal sequence, the (NANP)4 passenger sequence and the MisL translocator β domain | nirB | 70 kDa | Bacterial surface | Anaerobiosis |
| pUC19-CSP | 3,886 | pUC19 | Truncated form of the CSP from P. falciparum (amino acids 20 to 381) | lac | 60 kDaa | Bacterial cytosol | Inducible by IPTG |
The expected molecular mass is not consistent with the electrophoretic pattern (66 kDa).
TABLE 2.
Oligonucleotides
| Name | Sequence | Orientation | Characteristic(s) |
|---|---|---|---|
| MisL-1 | 5′ GGC TAA GCT AGC CAT GAA GAT GGC GAA CCA TGG 3′ | Sense | Contains the MisL bp-1348 to -1369 strand and an NheI restriction site (underlined) |
| MisL-2 | 5′ GCT AAG TCT AGA TCA GAA ACT GTA TTT CAT CCC CAG 3′ | Antisense | Contains the MisL bp 2844 to 2868 and an XbaI restriction site (underlined) |
| Nanp-1 | 5′ CT AGA CCA AAC GCT AAT CCT AAC GCC AAT CCA AAC GCA AAT CCT AAC GCT AAT CCA AAC GCT AGC ATG CAT G 3′ | Sense | Contains 4 repeats of the NANP epitope and XbaI NheI NsiI, and BamHI restriction sites (underlined) |
| Nanp-2 | 5′ GA TCC ATG CAT GCT AGC GTT TGG ATT AGC GTT AGG ATT TGC GTT TGG ATT GGC GTT AGG ATT AGC GTT TGG T 3′ | Antisense | Contains 4 repeats of the NANP epitope and BamHI, NsiI, NheI, and XbaI restriction sites (underlined) |
| pNir1 | 5′ TTCAGGTAAATTTGATACATCAAA 3′ | Sense | Contains a nirB promoter sequence |
Culture and induction conditions.
Bacterial strains transformed with plasmid pnirBMisL-NANP, pnirBMisL, or pUC19-CSP were cultured in brain heart infusion agar plates at 37°C with 100 μg of ampicillin/ml. Salmonella strains were supplemented with 0.01% 2,3-dihydroxybenzoic acid (DHB) (Sigma, St. Louis, Mo.). In order to induce the nirB promoter, a single colony was inoculated in brain heart infusion broth supplemented with ampicillin and DHB, as necessary. Once the culture reached late logarithmic growth phase (optical density at 600 nm [OD600] = 1.0), 150 μl (1.5 × 108 cells) was transferred to 50 ml of thioglycolate medium (Difco Laboratories, Detroit, Mich.) supplemented with antibiotic and DHB and incubated anaerobically at 37°C in a BBL GasPak Anaerobic System (Becton Dickinson Microbiology Systems, Cockeysville, Md.) until an OD600 of 1.0 was reached (approximately 18 h). Bacterial strains transformed with plasmid pUC19-CSP were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h at 37°C in aerobic conditions.
Genetic engineering.
DNA handling was performed according to the methods recommended by Sambrook and Russell (32). Plasmid pnirBMisL-NANP, containing the fusion protein MisLβ-(NANP)4, was constructed as follows. The MisL β domain was first predicted by alignment with AIDA-I (25) and then amplified by PCR from serovar Typhimurium SL3261 DNA according to the reported sequence (GenBank accession no. AF106566; S. enterica serovar Typhimurium pathogenicity island SPI-3; complete sequence gi|4324606|gb|F106566.1|F106566 4324606). Two primers were designed, sense MisL-1 (5′ GGC TAA GCT AGC CAT GAA GAT GGC GAA CCA TGG 3′) and antisense MisL-2 (5′ GCT AAG TCT AGA TCA GAA ACT GTA TTT CAT CCC CAG 3′), containing NheI and XbaI restriction sites (underlined), and the MisL-encoding sequence (bp 1348 to 1369 in MisL-1 and bp 2844 to 2868 in MisL-2). The PCR product (1,544 bp; amino acids 450 to 955) was inserted in plasmid pnirBLTBsp; the resulting plasmid (pnirBMisL) was digested with NheI/BamHI, releasing a 1,318-bp MisL β fragment; and the 2,627-bp fragment was ligated to the following oligonucleotides containing the (NANP)4-encoding sequence: sense Nanp-1 (5′ CT AGA CCA AAC GCT AAT CCT AAC GCC AAT CCA AAC GCA AAT CCT AAC GCT AAT CCA AAC GCT AGC ATG CAT G 3′) and antisense Nanp-2 (5′ GA TCC ATG CAT GCT AGC GTT TGG ATT AGC GTT AGG ATT TGC GTT TGG ATT GGC GTT AGG ATT AGC GTT TGG T 3′) containing XbaI, NheI, NsiI, and BamHI restriction sites (underlined) and four repeats of the tetrapeptide NANP. The resulting plasmid (pnirB-NANP) was digested with BamHI/NheI, the MisL β 1,318-bp fragment was reinserted, and correct clones were identified by the loss of the NsiI restriction site. Finally, the clones were sequenced. Briefly, 1 μg of pnirBMisL-NANP DNA was sequenced with the DNA Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, Calif.) with 8 pM pNir1 primer (5′TTCAGGTAAATTTGATACATCAAA). The amplification reaction mixture was purified with Centrisep Spin columns (Applied Biosystems) and examined in a PE ABI Prism 310 genetic analyzer (Applied Biosystems).
Bacterial fractions.
Different bacterial fractions were obtained from E coli DH5α transformed with pnirBLTBsp (E. coli-LTBsp), pnirBMisL (E. coli-MisL), pnirBMisL-NANP (E. coli-MisL-NANP), and pUC19-CSP (E. coli-CSP). One hundred milliliters of cultures grown under inducing conditions was harvested by centrifugation at 5,000 × g for 5 min, washed twice with phosphate-buffered saline (PBS; pH 7.4), and adjusted to 109 cells/ml (OD600 = 1.0). Crude protein extracts were obtained by mixing 108 cells with 100 μl of sample buffer (0.5 M Tris [pH 6.8], 2% sodium dodecyl sulfate [SDS], 5% β-mercaptoethanol, 10% glycerol, and 0.1% bromophenol blue), boiling the mixture for 10 min, and collecting the supernatant after centrifugation at 21,000 × g for 2 min. In order to obtain periplasmic proteins, 1010 cells were resuspended in 10 ml of 30 mM Tris-HCl with 20% sucrose (pH 8.0) in 1 mM EDTA, the cells were then incubated with agitation for 10 min at room temperature, the suspension was centrifuged at 8,000 × g for 10 min, and the pellet was resuspended in 300 μl of cold 5 mM MgSO4 and shaken on ice for 10 min. The suspension was centrifuged at 8,000 × g for 10 min at 4°C, and the supernatant containing the periplasmic fraction was collected. In order to obtain outer membrane proteins (OMP), 1010 cells were resuspended in 10 ml of 10 mM Tris-HCl (pH 8.0)-10 mM EDTA and disrupted in a French press (20,000 lb/in2, twice). Undisrupted cells were removed by centrifugation, and the supernatant was incubated with 1% Triton X-100 for 30 min and centrifuged at 100,000 × g (Beckman Optima XL-100K Ultracentrifuge) for 60 min at 4°C. The pellet containing OMP was dissolved in 10 mM Tris-EDTA. The protein concentration was quantified in all bacterial fractions (4), and samples were further analyzed by electrophoresis under reducing conditions.
Expression of the fusion protein MisLβ-(NANP)4 and Western blot analysis.
Expression of the fusion MisLβ-(NANP)4 protein was evaluated in different bacterial fractions. Protein samples were electrophoresed under reducing and denaturing conditions (1% SDS, 140 mM 2-mercaptoethanol, 95°C, 10 min) in 10% polyacrylamide gels (SDS-polyacrylamide gel electrophoresis ) according to the method of Laemmli (21) and then stained with Coomassie blue dye or transferred to nylon membranes (Millipore, Bedford, Mass.). Western blotting of the fusion protein was performed with monoclonal antibody 2A10, which recognizes the NANP epitope from P. falciparum (kindly donated by Elizabeth Nardin, Department of Medical and Molecular Parasitology, New York University School of Medicine, New York, N.Y.); 2 μg of monoclonal antibody/ml was diluted in PBS-5% skim milk. After washing, a goat anti-mouse IgG-peroxidase conjugate (1 μg/ml) was applied. The immune reaction was revealed with 4-chloro-1-naphthol-30% H2O2 in PBS (pH 7.4).
Immunofluorescence microscopy and flow cytometry.
Production of the MisLβ-(NANP)4 fusion protein was evaluated in E. coli DH5α, serovar Typhimurium SL3261, and serovar Typhi CVD 908 transformed with plasmid pnirBMisL, pnirBMisL-NANP, or pUC19-CSP by indirect immunofluorescence assay (IFA) and flow cytometry: After induction, the bacterial strains were harvested, washed twice with PBS, and incubated with monoclonal antibody 2A10 (2 μg/ml) in PBS plus 1% bovine serum albumin for 30 min at room temperature with agitation. They were washed twice with PBS and incubated with a goat anti-mouse IgG-fluorescein isothiocyanate (FITC) conjugate (10 μg/ml) for 30 min at room temperature in the dark. The washings were repeated, and the samples were resuspended in propidium iodide-PBS (4 mg/liter) and analyzed in a flow cytometer (30, 35) (Becton Dickinson, Bedford, Mass.) or spread on slides for viewing by fluorescence microscopy.
Animals and immunization schedules.
Female BALB/c mice, 9 to 12 weeks old (kindly donated by Ingeborg Becker, Unidad de Investigación Biomédica, Universidad Nacional Autónoma de México, Mexico City, Mexico), were bred and maintained in the Hospital General de Mexico. All animals were maintained and treated according to local and federal regulations. This work was approved by the Local Research and Bioethics Committee (reg. no. 2000-693-012). Groups of five mice were immunized with serovar Typhi CVD 908 transformed with pnirBMisL (serovar Typhi CVD 908-MisL), pnirBMisL-NANP (serovar Typhi CVD 908-MisL-NANP), or pUC19-CSP (serovar Typhi CVD 908-CSP) or with serovar Typhimurium SL3261 transformed with pnirBMisL (serovar Typhimurium SL3261-MisL), pnirBMisL-NANP (serovar Typhimurium SL3261-MisL-NANP), or pUC19-CSP (serovar Typhimurium SL3261-CSP). All bacterial strains were cultured under inducing conditions, harvested in late logarithmic growth phase, and adjusted to 1010 cells/ml. Serovar Typhi CVD 908 derivatives were inoculated intranasally (the animals were inoculated with two successive 20-μl applications) (7), and serovar Typhimurium SL3261 was administered intragastrically; the animals received 200 μl with a 22-gauge feeder. Mice received four doses with intervals of 15 days and were maintained with 0.6 mg of ampicillin/ml in the drinking water.
Antibodies against NANP.
Sera from immunized mice were collected on days 0 and 65. Specific antibodies against NANP were determined by enzyme-linked immunosorbent assay (ELISA), blindly for each individual mouse (37). Flat-bottomed-well microtiter plates (Nunc) were coated with 5 μg of a NANP synthetic peptide (kindly donated by Vianney Ortiz, Departamento de Biomedicina Molecular, CINVESTAV, Instituto Politécnico Nacional, Mexico City, Mexico)/ml in 200 μl of carbonate buffer for 2 h at 37°C and overnight at 4°C. Then, the plates were blocked with 5% skim milk-PBS and incubated with serial dilutions (1:10 to 1:320) of the sera for 2 h at 37°C. A goat anti-mouse IgG-peroxidase conjugate (0.5 μg/ml) was added and incubated for 2 h at 37°C, and color was developed with o-phenylenediamine-H2O2 in PBS. The reaction was arrested with 50 μl of 2.5 N H2SO4, and the OD490 was determined (ELX808; Bio-Tek Instruments). Between incubations the plates were washed three times with PBS-0.1% Tween 20.
Recognition of native CSP in P. falciparum.
Sera from those mice with antibodies against NANP determined by ELISA were further analyzed to assess their ability to recognize P. falciparum by IFA. Slides containing fixed P. falciparum sporozoites were incubated with 1:2 dilutions of sera in a humid chamber for 1 h, followed by five washes with PBS-0.1% Tween 20 and one wash with PBS. The slides were incubated with an anti-mouse IgG-FITC conjugate (10 μg/ml) for 30 min, the washings were repeated, and the slides were observed in a fluorescence microscope (BX-40; Olympus).
Statistical analysis.
Intragroup and intergroup statistical analyses were performed with nonparametric Kruskal-Wallis analysis of variance, which allowed us to make post hoc comparisons with the Mann-Whitney U test, considering differences as significant when P was ≤0.01.
RESULTS
Construction of the MisLβ-(NANP)4 fusion protein.
The predicted MisL amino acid sequence was aligned with the reported topologic model of AIDA-I (25). The proteins showed 43% conserved amino acids in the C-terminal region corresponding to the translocator β domain. The MisL topologic model of the β barrel was very similar to AIDA-I, revealing amino acid differences located mainly in the external loops and internal turns, whereas the β-sheet regions were highly conserved (Fig. 1).
FIG. 1.
Alignment of MisL and AIDA-I β translocator domains. Alignment of the C-terminal sequence of AIDA-I and MisL, performed with the seq-LALIGN version 2.0u66 software. Amino acids 661 to 955 of the MisL β domain are presented, although the sequence corresponding to amino acids 450 to 955 was later cloned. Solid black boxes, identical amino acids; solid gray boxes, conserved amino acids; no shading, unconserved amino acids; β, transmembranal domain; L, loop toward outside; T, turn toward inside.
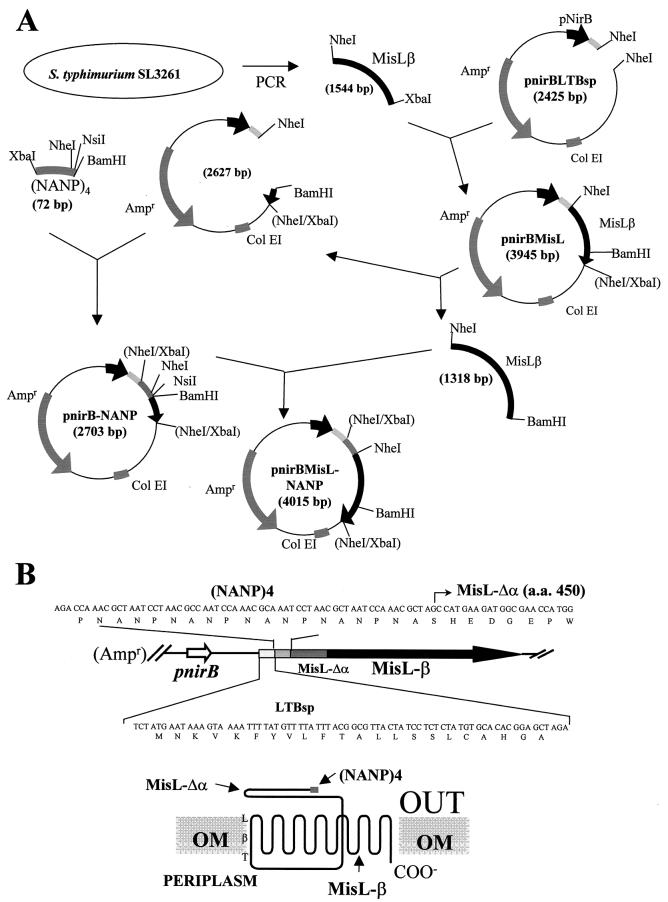
Plasmid pnirBMisL-NANP containing a hybrid gene under the control of the anaerobically inducible nirB promoter, which allows efficient expression of recombinant antigens in live bacterial vectors, was constructed as depicted in Fig. 2. In the construction of the synthetic gene, the LTB signal peptide was inserted in order to direct the fusion protein translocation to the periplasmic space in a sec-dependent manner; this sequence was followed by four repeats of the tetrapeptide NANP, the main B-cell epitope of CSP. This 16-amino-acid sequence was placed upstream and in frame with the MisL β domain. The putative MisL β domain included an additional 163 amino acids in the N-terminal end in order to ensure transit of the passenger domain through the pore and avoid possible misorientation toward the periplasmic space or embedding at the pore (Fig. 2B).
FIG. 2.
Construction of plasmid pnirBMisL-NANP (4,015 bp), which contains a fusion synthetic gene encoding the LTB signal sequence, the (NANP)4 passenger sequence, and the MisL translocator β domain under the control of the nirB promoter.
The MisLβ-(NANP)4 fusion protein reaches the bacterial outer membrane.
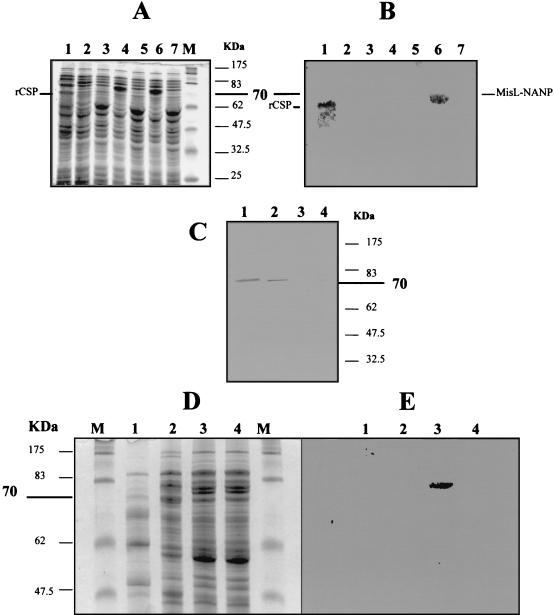
Expression of the MisLβ-(NANP)4 fusion protein was evaluated by SDS-PAGE and Western blotting in different bacterial fractions purified from E. coli-MisL or E. coli-MisL-NANP cultured in aerobic and anaerobic conditions. Controls included E. coli-LTBsp or E. coli-CSP. The electrophoretic pattern of total protein extracts showed overexpression of an ∼70-kDa protein in E. coli-MisL and E. coli-MisL-NANP, but not in E. coli-LTBsp, when the strains were cultured anaerobically (Fig. 3A). Expression of the NANP epitope was confirmed by Western blotting only for E. coli-MisL-NANP and E. coli-CSP (cultures in aerobic conditions and induced with IPTG) (Fig. 3B). Electrophoresis of periplasmic fractions failed to demonstrate clear protein overexpression on a stained gel (data not shown). Nevertheless, Western blot analysis of the periplasmic samples confirmed the presence of the fusion protein only in E. coli-MisL-NANP. Interestingly, the detection of fusion protein in periplasmic fractions required 10 times more bacteria than the amount for OMP (Fig. 3C), suggesting transient passage of MisLβ-(NANP)4 through the periplasmic space and rapid association with the external membrane. Electrophoresis of OMP extracts showed a band with the expected size (∼70 kDa) in E. coli-MisL and E. coli-MisL-NANP (Fig. 3D) but NANP expression only in E. coli-MisL-NANP (Fig. 3E). As expected, the NANP epitope was found in total bacterial extracts from E. coli-CSP but not in periplasmic or in OMP fractions, because this strain produces a cytosolic form of the protein, thus demonstrating that the bacterial fractionation was appropriate and that the passenger peptide translocation to the periplasmic or outer membrane compartments was MisL dependent and not an experimental artifact.
FIG. 3.
Expression of the MisLβ-(NANP)4 fusion protein in different bacterial extracts from E. coli DH5α. (A) SDS-PAGE of total protein extracts from E. coli transformed with different plasmids. Lanes: 1, pUC19-CSP, cultured aerobically with IPTG; 2, pnirBLTBsp, cultured anaerobically; 3, pnirBLTBsp, cultured aerobically; 4, pnirBMisL, cultured anaerobically; 5, pnirBMisL, cultured aerobically; 6, pnirBMisL-NANP, cultured anaerobically; 7, pnirBMisL-NANP, cultured aerobically. (B) Western blot of same samples as for panel A. (C) Western blot of periplasmic extracts revealed with 2A10 antibody against NANP. Lanes: 1 and 2, pnirBMisL-NANP, cultured anaerobically; 3, pnirBMisL, cultured anaerobically; 4, pUC19-CSP, cultured aerobically with IPTG. (D) SDS-PAGE of OMP. Lanes: 1, pUC19-CSP, cultured aerobically with IPTG; 2, pnirBLTBsp, cultured anaerobically; 3, pnirBMisL-NANP, cultured anaerobically; 4, pnirBMisL, cultured anaerobically. (E) Western blot of OMP revealed with 2A10 antibody against NANP with the same samples as for panel D. Lanes M, molecular mass markers.
The (NANP)4 passenger peptide is expressed on the bacterial surface.
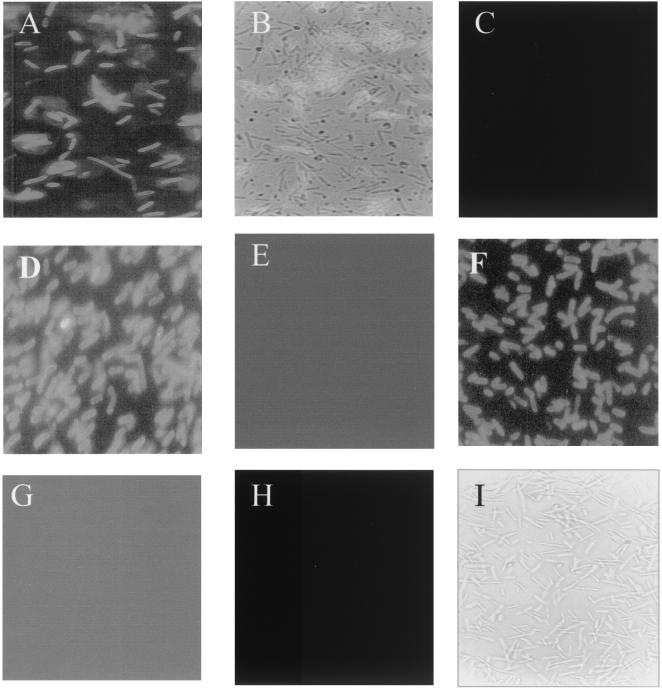
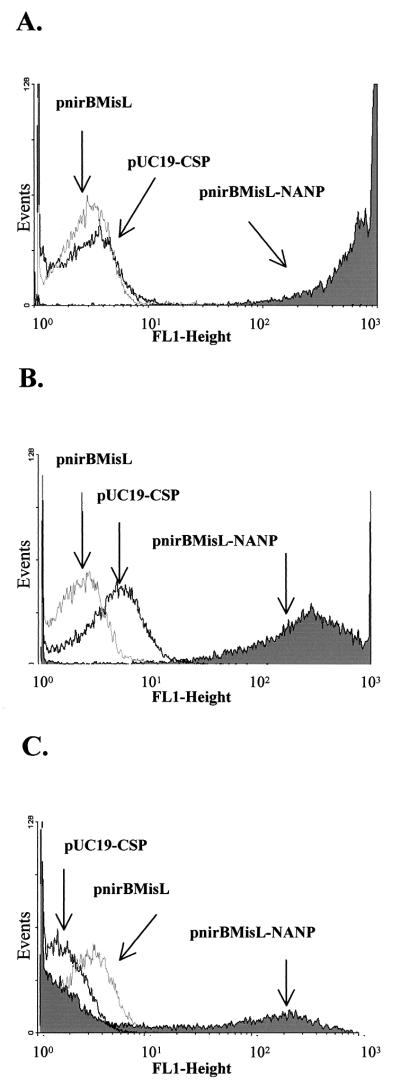
The ability of the recombinant MisL β domain to translocate the (NANP)4 passenger peptide to the bacterial surface was traced by IFA and flow cytometry with the 2A10 antibody and an anti-mouse IgG-FITC conjugate. E. coli DH5α, serovar Typhi CVD 908, and serovar Typhimurium SL3261 were transformed with pnirBMisL, pnirBMisL, or pUC19-CSP. Figure 4 shows that the NANP peptide is expressed on the surface of E. coli-MisL-NANP and Salmonella MisL-NANP (Fig. 4A, B, D, and F). Serovar Typhi CVD 908-CSP, producing a cytosolic form of CSP, did not show fluorescence, demonstrating that under the experimental conditions the cells were not permeable (Fig. 4H and I). Further analysis by flow cytometry demonstrated distinct fusion protein expression among the different transformed strains (Fig. 5). E. coli-MisL-NANP and serovar Typhimurium SL3261-MisL-NANP showed high expression of the NANP epitope; both strains reached 2-log-relative-light-unit displacement with respect to the controls (Fig. 5A and B). In contrast, serovar Typhi CVD 908-MisL-NANP seems to display two populations, one expressing the NANP epitope and the other not expressing it (Fig. 5C). Nevertheless, the positive population also reaches 2-log-relative-light-unit displacement with respect to its controls. This behavior may be related to the low growth rate in this aroC aroD mutant and a possible tendency to lose the plasmid. Nevertheless, the results obtained by IFA and flow cytometry indicate that the NANP epitope translocation depended on the MisL β domain and suggest that it could be applied to other gram-negative bacteria
FIG. 4.
Expression of the P. falciparum NANP epitope on the bacterial surface of E. coli DH5α, serovar Typhimurium SL3261, and serovar Typhi CVD 908 cultured under anaerobic conditions and determined by IFA with 2A10 monoclonal antibody against NANP. (A) E. coli DH5α transformed with pnirBMisL-NANP; (B) same strain as that in panel A, contrast enhanced with visible light; (C) E. coli DH5α transformed with pnirBMisL; (D) serovar Typhimurium SL3261 transformed with pnirBMisL-NANP; (E) serovar Typhimurium SL3261 transformed with pnirBMisL; (F) serovar Typhi CVD 908 transformed with pnirBMisL-NANP; (G) serovar Typhi CVD 908 transformed with pnirBMisL; (H) E. coli DH5α transformed with pUC19-CSP; (I) same strain as that in panel H, contrast enhanced with visible light.
FIG. 5.
Flow cytometry analysis of the P. falciparum NANP epitope expression on the surface of different bacterial strains. Bacterial surface expression of the passenger epitope was assessed with the monoclonal antibody 2A10, which recognizes the P. falciparum NANP epitope, and then revealed with a goat anti-mouse IgG-FITC conjugate. (A) E. coli DH5α transformed with pUC19-CSP, pnirBMisL, or pnirBMisL-NANP; (B) serovar Typhimurium SL3261 transformed with the same plasmids; (C) serovar Typhi CVD 908 transformed with the same plasmids.
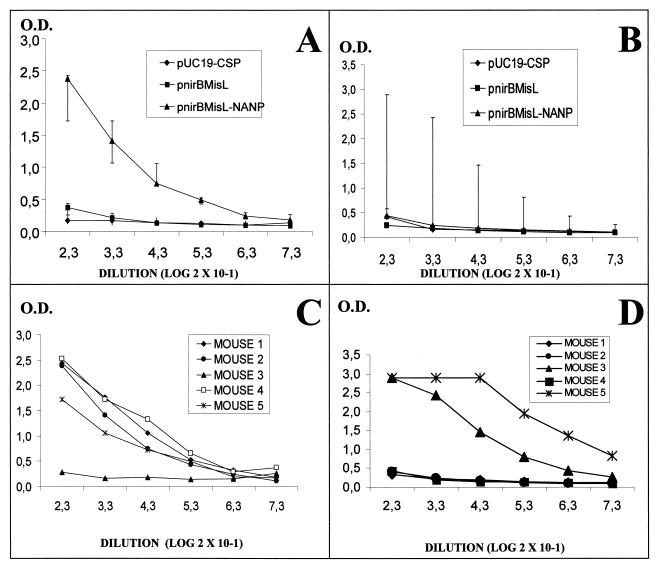
The (NANP)4 passenger peptide displayed on the Salmonella surface elicits antibodies.
In order to determine whether the (NANP)4 passenger antigen was able to elicit antibodies, groups of BALB/c mice were immunized intragastrically with serovar Typhimurium SL3261-MisL-NANP or intranasally with serovar Typhi CVD 908-MisL-NANP. The mice received four doses of 1010 CFU with intervals of 15 days, and the antibody titers were measured by ELISA with a synthetic peptide bearing the NANP sequence. Serovar Typhimurium SL3261-MisL-NANP elicited an intense antibody response in four out of five immunized mice (Fig. 6A and C). In contrast, mice immunized with serovar Typhi CVD 908-MisL-NANP displayed more dispersed behavior: only two out of five animals presented clear antibody production against the NANP epitope (Fig. 6B and D). This result was consistent with the serovar Typhi CVD 908 behavior in the flow cytometry experiments. Neither serovar Typhimurium SL3261 nor serovar Typhi CVD 908 transformed with plasmid pUC19-CSP, which produced a cytosolic recombinant protein, was able to elicit antibodies against the NANP epitope.
FIG. 6.
Antibodies against the P. falciparum NANP epitope elicited by serovar Typhimurim SL3261 or serovar Typhi CVD 908 expressing the MisLβ-(NANP)4 fusion protein. Antibodies were determined by ELISA against a NANP synthetic peptide. (A) Groups of mice immunized with serovar Typhimurium SL3261 transformed with pUC19-CSP, pnirBMisL, or pnirBMisL-NANP; (B) groups of mice immunized with serovar Typhi CVD 908 transformed with the same plasmids; (C) individual mouse response to serovar Typhimurium SL3261 transformed with pnirBMisL-NANP; (D) individual mouse response to serovar Typhi CVD 908 transformed with pnirBMisL-NANP.
Antibodies against the (NANP)4 passenger peptide are able to recognize native CSP in P. falciparum.
The ability of antibodies raised by the NANP epitope on the Salmonella surface to recognize native CSP in P. falciparum was further analyzed by IFA. Sera from mice immunized with serovar Typhi CVD 908-MisL-NANP and positive by ELISA were evaluated with slides containing P. falciparum sporozoites. Figure 7A demonstrates that serovar Typhi CVD 908-MisL-NANP elicited antibodies which recognized the NANP epitope in native CSP. In contrast, sera from mice immunized with the control strain, expressing only MisL, were not able to recognize the sporozoite (Fig. 7B). These results demonstrate that the NANP epitope on the bacterial surface maintained its conformational and immunogenic properties and elicited antibodies which reacted with the parasite.
FIG. 7.
Antibodies elicited by the MisLβ-(NANP)4 fusion protein recognize the NANP epitope in P. falciparum. The figure shows P. falciparum sporozoites revealed by IFA with sera from mice immunized with serovar Typhi CVD 908 transformed with pnirBMisL-NANP (A) or with sera from mice immunized with serovar Typhi CVD 908 transformed with pnirBMisL (B).
DISCUSSION
Autotransporters may be divided into two subgroups according to the complexity of their translocator β domain (10). In classic autotransporters the β barrel is formed by 14 β sheets, which form seven transmembrane regions; whereas nonclassic autotransporters contain more transmembrane β sheets, forming a wider pore. MisL may be considered a classic autotransporter with a structure similar to that of AIDA-I. Alignment of the C terminus of MisL with that of AIDA-I (2) demonstrated approximately 43% conserved amino acids and, moreover, 55% with a similar hydrophobic nature. This high degree of homology allowed us to assume a hypothetical topologic model for MisL based on the known structure of the AIDA-I C terminus and to select the putative β domain with the linker region which allows the passenger domain to transit through the pore to the bacterial surface.
Herein we report the construction of a hybrid protein containing the LTB signal peptide, four repeats of the NANP tetrapeptide, and the MisL β domain, which allowed expression of the passenger peptide on the surface of S. enterica. Protein expression was directed by the plasmid-encoded gene driven by the anaerobically inducible nirB promoter, which may have some advantages over other promoters. It induces strong recombinant protein expression in vivo, when the bacteria find a microaerophilic or acid environment in the intestine and in the phagolysosome, once it is taken up by macrophages (5)
Although the MisL natural trafficking signal is unknown, the enterotoxigenic E. coli LTB leader sequence was chosen because MisL transit to the periplasmic compartment, as for other autotransporters, must be sec dependent, and we have previously obtained periplasmic protein expression by using this signal sequence (unpublished results). Interestingly, the linker peptide, which plays an essential role in driving the α passenger domain though the β domain pore, also displayed high homology to AIDA-I. A 163-amino-acid linker strand was included in order to ensure correct passenger peptide translocation. According to the alignment model, this amino acid strand could be shorter, and it may be possible to truncate it to obtain the minimal functional length. It has been reported previously that the minimal essential linking region in AIDA-I contains 28 to 48 amino acids (26). Four repeats of the tetrapeptide NANP were inserted behind the LTB leader sequence, and expression of the resulting MisLβ-(NANP)4 hybrid protein was evaluated in E. coli DH5α, serovar Typhimurium SL3261, and serovar Typhi CVD 908. All of these bacterial strains were able to produce the recombinant protein as confirmed by SDS-PAGE and Western blotting and to translocate the passenger peptide to the bacterial surface as demonstrated by IFA and flow cytometry. However, serovar Typhimurium exhibited better NANP expression than did serovar Typhi CVD 908. The low expression efficiency of the recombinant protein in serovar Typhi could be related to plasmid stability, but further analysis is necessary to elucidate this difference. Moreover, although the MisLβ-(NANP)4 fusion protein did not produce an appreciable decrease in viability, it is not possible to establish what bacterial functions might be altered, because the MisL biological role is still unknown.
The central area of the CSP is formed by the tandemly repeated sequence of NANP amino acids. In adults in areas of endemicity, a serologic response to P. falciparum sporozoites is found against the NANP epitope, after prolonged exposure to the malaria parasite. The reason for this immunodominance may simply be that NANP is abundantly represented on the sporozoite surface.
The safety and immunogenicity of a parenterally administered NANP synthetic peptide in humans have been demonstrated previously (14). When the entire CSP antigen was expressed in the cytoplasm of serovar Typhimurium, immunized mice responded with cellular responses and were protected against challenge, but no antibody production occurred (31). In clinical trials vaccination with serovar Typhi expressing a cytoplasmically located fragment of CSP induced antibody responses in only 30% of vaccinees (8). We hypothesized that cytoplasmic localization and antigen concentration were inadequate for induction of protective responses. Studies by Hess and coworkers demonstrated the importance of the antigen expression site in a heterologous host strain when secreted p60 or listeriolysin (Hly) antigen was able to induce protection against listeriosis while the cytoplasmically expressed derivative was not (15). Here we report that surface expression of the NANP epitope was crucial for eliciting specific antibody responses. In both serovar Typhimurium and serovar Typhi, only the surface-expressed MisL-NANP fusion, not the cytoplasmically expressed CSP construct, was able to direct production of antibodies in mice. Furthermore, these antibodies were able to detect native CSP in P. falciparum sporozoites. An additional finding was that, while the serovar Typhi live vector elicited antibody responses to the NANP epitope in only two of the five immunized mice compared to four of five mice for the serovar Typhimurium vector, the responses in the two serovar Typhi-immunized mice were higher than those in the serovar Typhimurium-immunized animals, thus demonstrating the promise of the use of serovar Typhi as a live-vector strain.
This bacterial display system may have other applications, which include ligand-receptor interaction studies, cellular signaling, recombinant protein production, and purification and specific antibody production.
Acknowledgments
We thank Vianney Ortiz, Ingeborg Becker, Leopoldo Santos, and Armando Isibasi for their technical assistance.
This work was in part supported by CONACYT grant 264100-5-5382 M, P.I., to C.G.-B. F.R.-P., A.S.-M., and R.L.-K. were recipients of a fellowship from CONACYT and IMSS. This research was also supported by NIH grant R01AI29471 to M.L.
Editor: A. D. O'Brien
REFERENCES
- 1.Al-Hasani, K., I. R. Henderson, H. Sakellaris, K. Rajakumar, T. Grant, J. P. Nataro, R. Robins-Browne, and B. Adler. 2000. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68:2457-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz, I., and M. A. Schmidt. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc-Potard, A. B., F. Solomon, J. Kayser, and E. A. Groisman. 1999. The SPI-3 pathogenicity island of Salmonella enterica. J. Bacteriol. 181:998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Chatfield, S. N., I. G. Charles, A. J. Makoff, M. D. Oxer, G. Dougan, D. Pickard, D. Slater, and N. F. Fairweather. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology 10:888-892. [DOI] [PubMed] [Google Scholar]
- 6.Dozois, C. M., M. Dho-Moulin, A. Bree, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galen, J. E., O. G. Gomez-Duarte, G. A. Losonsky, J. L. Halpern, C. S. Lauderbaugh, S. Kaintuck, M. K. Reymann, and M. M. Levine. 1997. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 15:700-708. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez, C., D. Hone, F. R. Noriega, C. O. Tacket, J. R. Davis, G. A. Losonsky, J. P. Nataro, S. Hoffman, A. Malik, E. Nardin, M. B. Sztein, D. G. Heppner, T. R. Fouts, A. Isibasi, and M. M. Levine. 1994. Salmonella typhi vaccine strain CVD 908 expressing the circumsporozoite protein of Plasmodium falciparum: strain construction and safety and immunogenicity. J. Infect. Dis. 169:927-931. [DOI] [PubMed] [Google Scholar]
- 9.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 10.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 11.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson, I. R., R. Cappello, and J. P. Nataro. 2000. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 8:529-532. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrington, D. A., D. F. Clyde, G. Losonsky, M. Cortesia, J. R. Murphy, J. Davis, S. Baqar, A. M. Felix, E. P. Heimer, D. Gillessen, E. Nardin, R. S. Nussenzweig, V. Nussenzweig, M. R. Hollingdale, and M. M. Levine. 1987. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328:257-259. [DOI] [PubMed] [Google Scholar]
- 15.Hess, J., I. Gentschev, D. Miko, M. Welzel, C. Ladel, W. Goebel, and S. H. E. Kaufmann. 1996. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc. Natl. Acad. Sci. USA 93:1458-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 17.Hone, D. M., A. M. Harris, S. Chatfield, G. Dougan, and M. M. Levine. 1991. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine 9:810-816. [DOI] [PubMed] [Google Scholar]
- 18.Kingsley, R. A., K. van Amsterdam, N. Kramer, and A. J. Baumler. 2000. The shdA gene is restricted to serotypes of Salmonella enterica subspecies I and contributes to efficient and prolonged fecal shedding. Infect. Immun. 68:2720-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klauser, T., J. Kramer, K. Otzelberger, J. Pohlner, and T. F. Meyer. 1993. Characterization of the Neisseria IgA beta-core. The essential unit for outer membrane targeting and extracellular protein secretion. J. Mol. Biol. 234:579-593. [DOI] [PubMed] [Google Scholar]
- 20.Konieczny, M. P., M. Suhr, A. Noll, I. B. Autenrieth, and S. M. Alexander. 2000. Cell surface presentation of recombinant (poly-)peptides including functional T-cell epitopes by the AIDA autotransporter system. FEMS Immunol. Med. Microbiol. 27:321-332. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lattemann, C. T., J. Maurer, E. Gerland, and T. F. Meyer. 2000. Autodisplay: functional display of active β-lactamase on the surface of Escherichia coli by the AIDA-I autotransporter. J. Bacteriol. 182:3726-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leininger, E., M. Roberts, J. G. Kenimer, I. G. Charles, N. Fairweather, P. Novotny, and M. J. Brennan. 1991. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc. Natl. Acad. Sci. USA 88:345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer, J., J. Jose, and T. F. Meyer. 1997. Autodisplay: one-component system for efficient surface display and release of soluble recombinant proteins from Escherichia coli. J. Bacteriol. 179:794-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer, J., J. Jose, and T. F. Meyer. 1999. Characterization of the essential transport function of the AIDA-I autotransporter and evidence supporting structural predictions. J. Bacteriol. 181:7014-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nussenzweig, V., and R. Nussenzweig. 1986. Experimental basis for the development of a synthetic vaccine against Plasmodium falciparum malaria sporozoites. Ciba Found. Symp. 119:150-163. [DOI] [PubMed] [Google Scholar]
- 28.Oxer, M. D., C. M. Bentley, J. G. Doyle, T. C. Peakman, I. G. Charles, and A. J. Makoff. 1991. High level heterologous expression in E. coli using the anaerobically-activated nirB promoter. Nucleic Acids Res. 19:2889-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohlner, J., R. Halter, K. Beyreuther, and T. F. Meyer. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458-462. [DOI] [PubMed] [Google Scholar]
- 30.Robertson, B. R., and D. K. Button. 1989. Characterizing aquatic bacteria according to population, cell size, and apparent DNA content by flow cytometry. Cytometry 10:70-76. [DOI] [PubMed] [Google Scholar]
- 31.Sadoff, J. C., W. R. Ballou, L. S. Baron, W. R. Majarian, R. N. Brey, W. Hockmeyer, J. F. Young, S. J. Cryz, J. Ou, G. H. Lowell, and J. D. Chulay. 1988. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science 240:336-338. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.St. Geme, J., III, and D. Cutter. 2000. The Haemophilus influenzae Hia adhesin is an autotransporter protein that remains uncleaved at the C terminus and fully cell associated. J. Bacteriol. 182:6005-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki, T., M. C. Lett, and C. Sasakawa. 1995. Extracellular transport of VirG protein in Shigella. J. Biol. Chem. 270:30874-30880. [DOI] [PubMed] [Google Scholar]
- 35.Van Der Waaij, L. A., G. Mesander, P. C. Limburg, and D. Van Der Waaij. 1994. Direct flow cytometry of anaerobic bacteria in human feces. Cytometry 16:270-279. [DOI] [PubMed] [Google Scholar]
- 36.Veiga, E., V. de Lorenzo, and L. A. Fernandez. 1999. Probing secretion and translocation of a beta-autotransporter using a reporter single-chain Fv as a cognate passenger domain. Mol. Microbiol. 33:1232-1243. [DOI] [PubMed] [Google Scholar]
- 37.Zavala, F., J. P. Tam, and A. Masuda. 1986. Synthetic peptides as antigens for the detection of humoral immunity to Plasmodium falciparum sporozoites. J. Immunol. Methods 93:55-61. [DOI] [PubMed] [Google Scholar]