Abstract
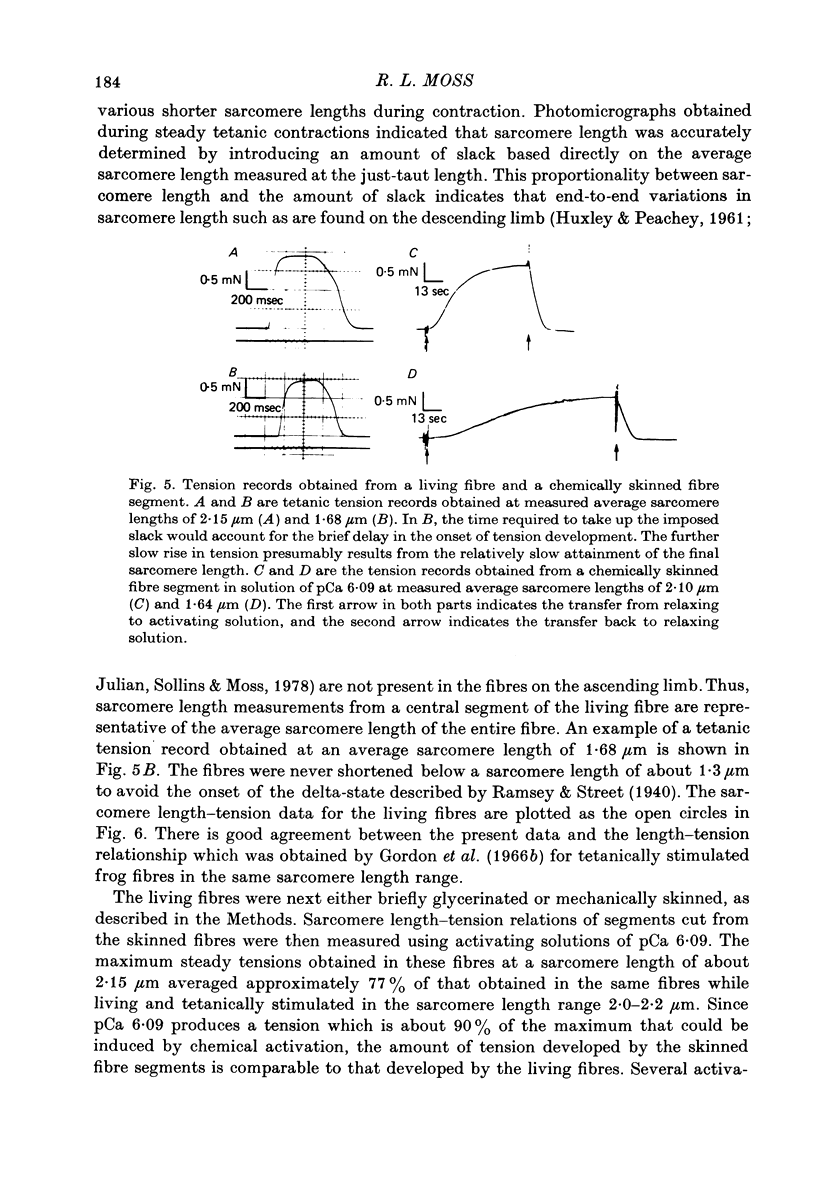
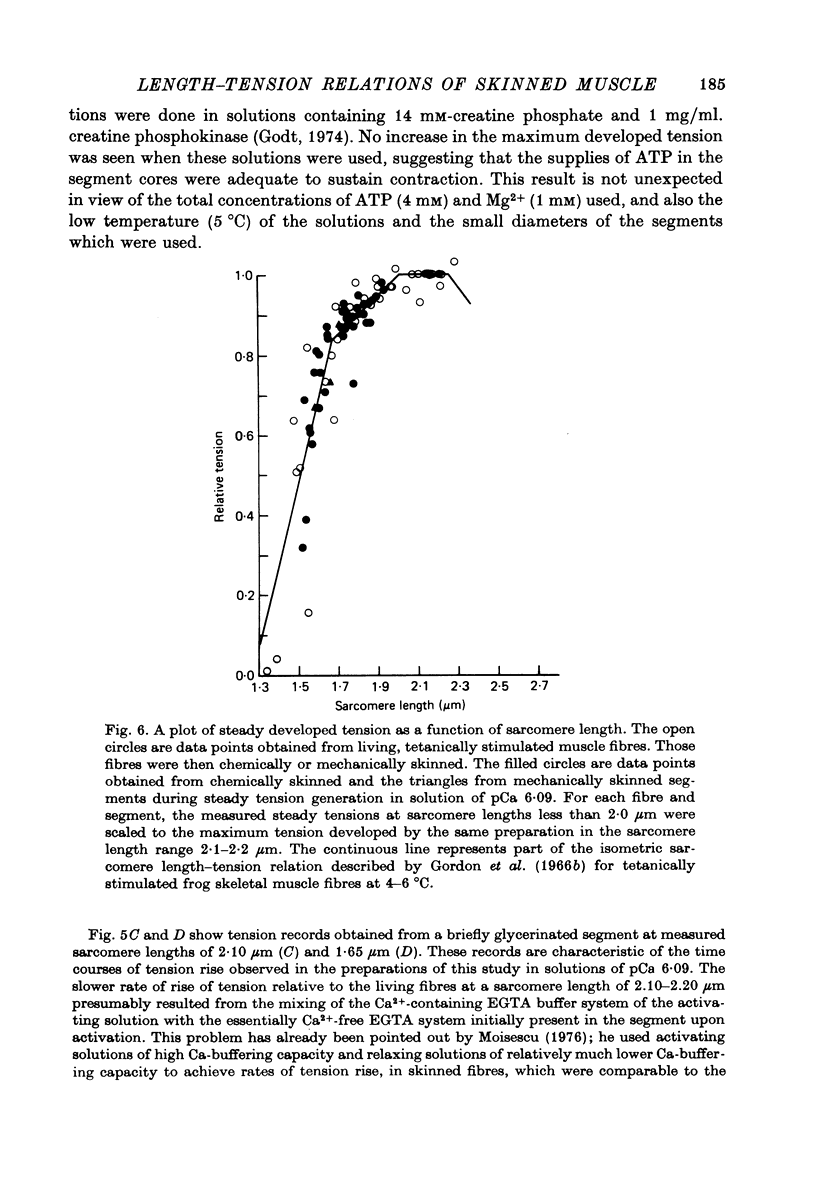
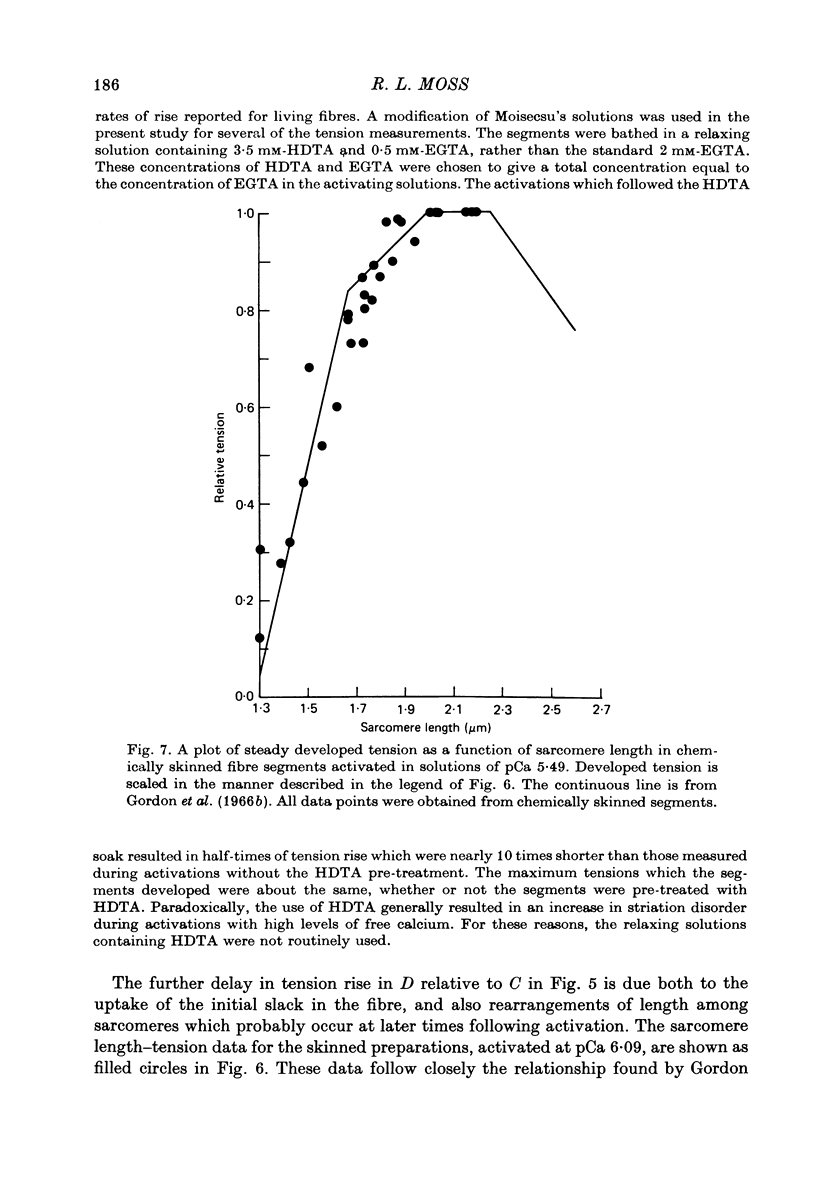
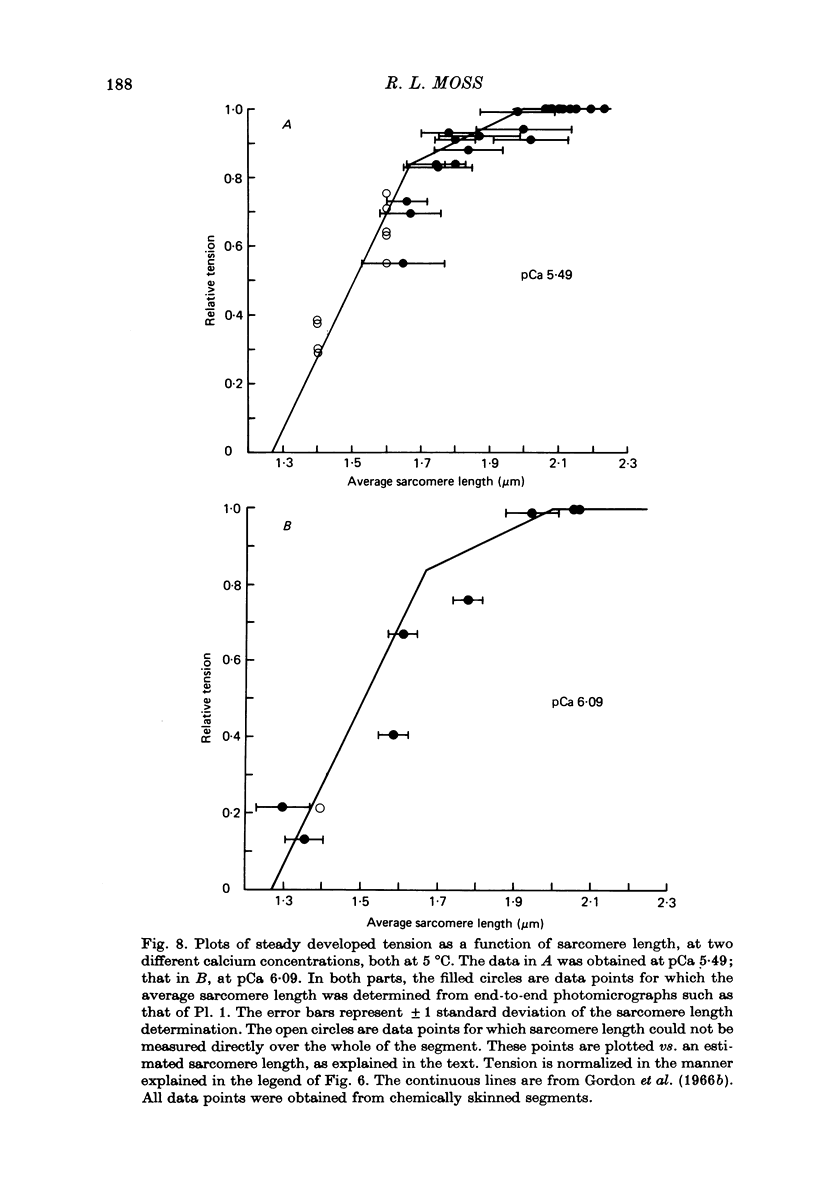
1. Single twitch fibres were isolated from anterior tibial muscles of the frog, Rana pipiens. The relationship between sarcomere length and steady tetanic tension at 5 degrees C was obtained from these living fibres in the range of sarcomere lengths between about 2.2 and 1.3 microns . These fibres were then either mechanically or chemically skinned. 2. Segments were cut from the skinned fibres and mounted in an experimental chamber using a technique designed to minimize segment compliance at the points of attachment. A piece approximately 1 mm in length remained exposed to the bathing solution. 3. The segments were photographed through a light microscope at magnifications of about 460 or 110 X during activation and relaxation, so that the sarcomere lengths could be determined from a part or the whole of the segment. Activations were done with solutions of pCa either 5.49 or 6.09 and at a temperature of 5 degrees C. Fibre segments which developed striation pattern irregularities during contraction were rejected. 4. The sarcomere length-tension relation obtained from these segments in the sarcomere length range 1.3-2.2 microns was similar to that obtained from the same fibres while still living. The results were similar at the two values of pCa used. 5. These results do not support the view that sarcomere length dependent variation in the amount of calcium which is released during tetanic stimulation is a major determinant of the form of the length-tension relation in living muscle fibres at sarcomere lengths less than about 2.0 microns.
Full text
PDF







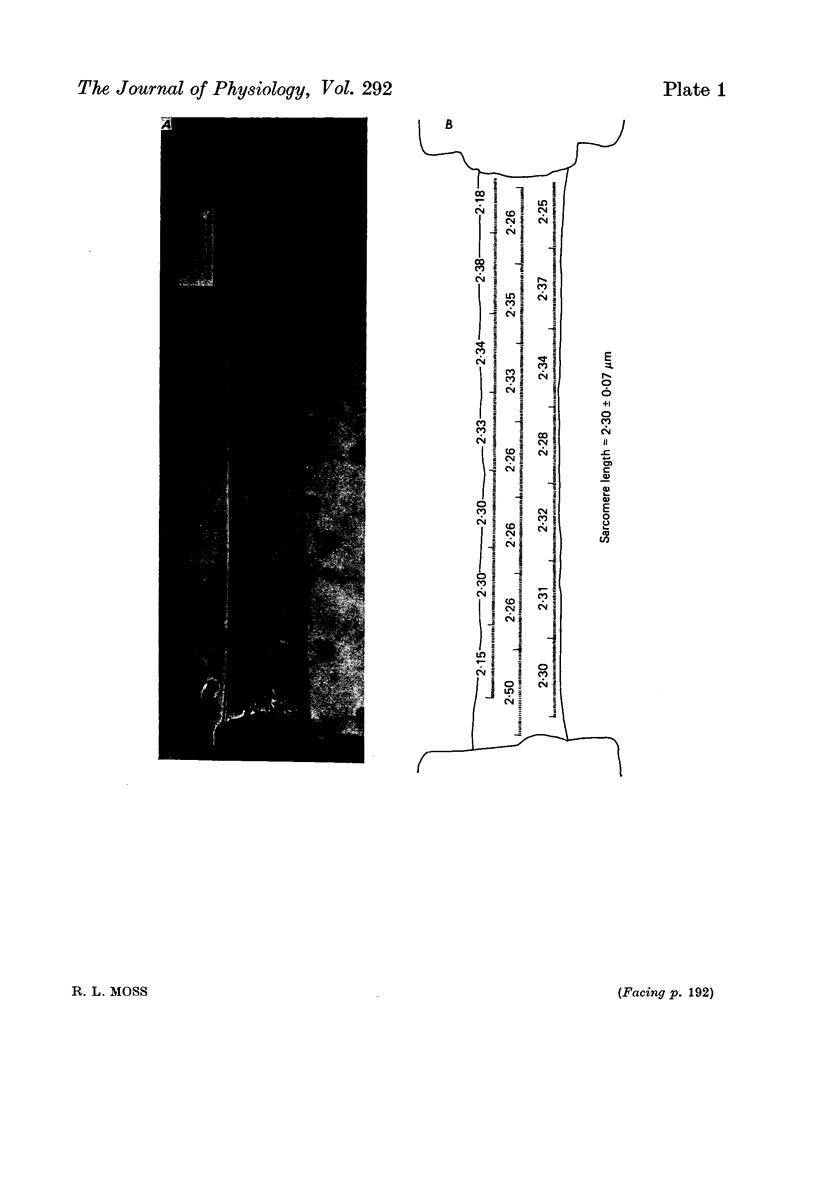

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E., Kean C. J. Skeletal muscle energetics and metabolism. Annu Rev Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- IWANAMI M., OSIBA S., YAMADA T., YOSHIMURA H. Seasonal variations in serum inorganic phosphate and calcium with special reference to parathyroid activity. J Physiol. 1959 Dec;149:23–33. doi: 10.1113/jphysiol.1959.sp006322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R., Moss R. L. Sarcomere length non-uniformity in relation to tetanic responses of stretched skeletal muscle fibres. Proc R Soc Lond B Biol Sci. 1978 Jan 24;200(1138):109–116. doi: 10.1098/rspb.1978.0009. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Variation of muscle stiffness with force at increasing speeds of shortening. J Gen Physiol. 1975 Sep;66(3):287–302. doi: 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Sollins M. R., Julian F. J. Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature. 1976 Apr 15;260(5552):619–621. doi: 10.1038/260619a0. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Taylor S. R. Striated muscle fibers: facilitation of contraction at short lengths by caffeine. Science. 1971 Apr 23;172(3981):387–389. doi: 10.1126/science.172.3981.387. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Podolsky R. J. Length-force relation of calcium activated muscle fibers. Science. 1972 Apr 7;176(4030):52–54. doi: 10.1126/science.176.4030.52. [DOI] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R. Striated muscle fibers: inactivation of contraction induced by shortening. Science. 1970 Feb 6;167(3919):882–884. doi: 10.1126/science.167.3919.882. [DOI] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]




