Abstract
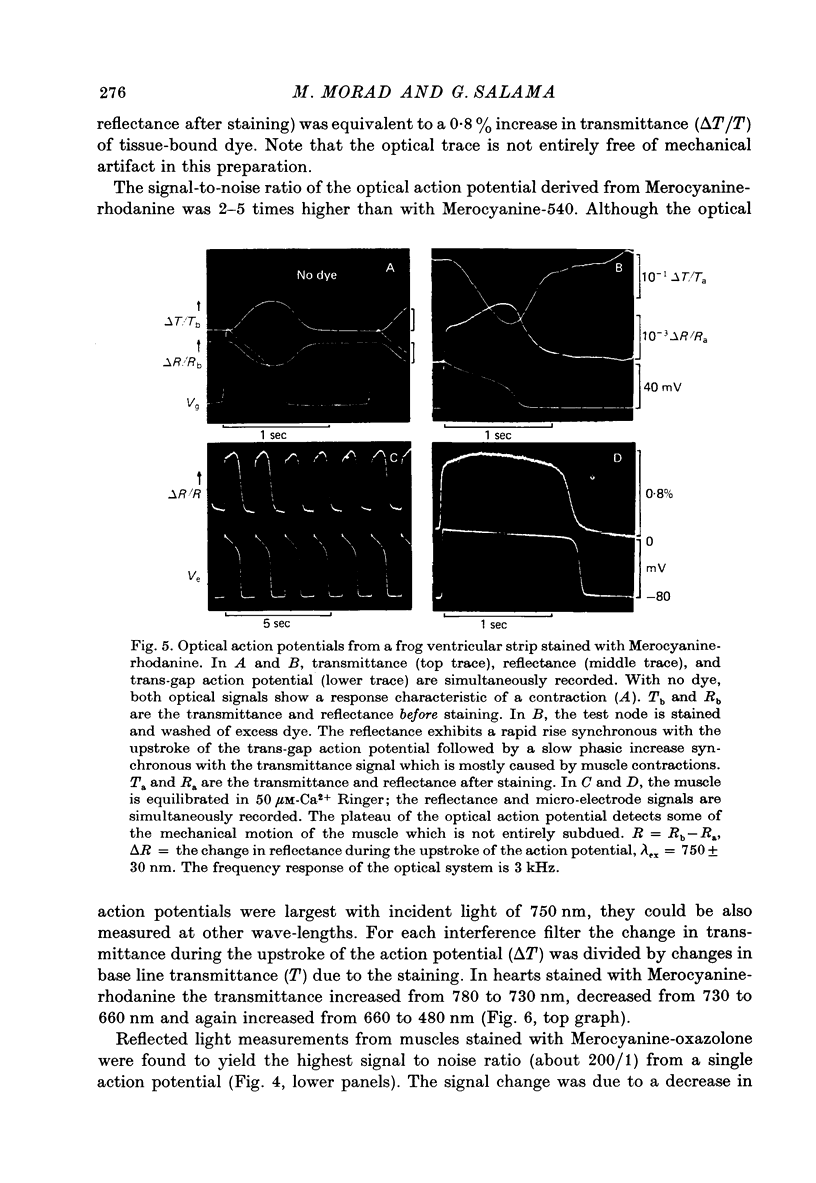
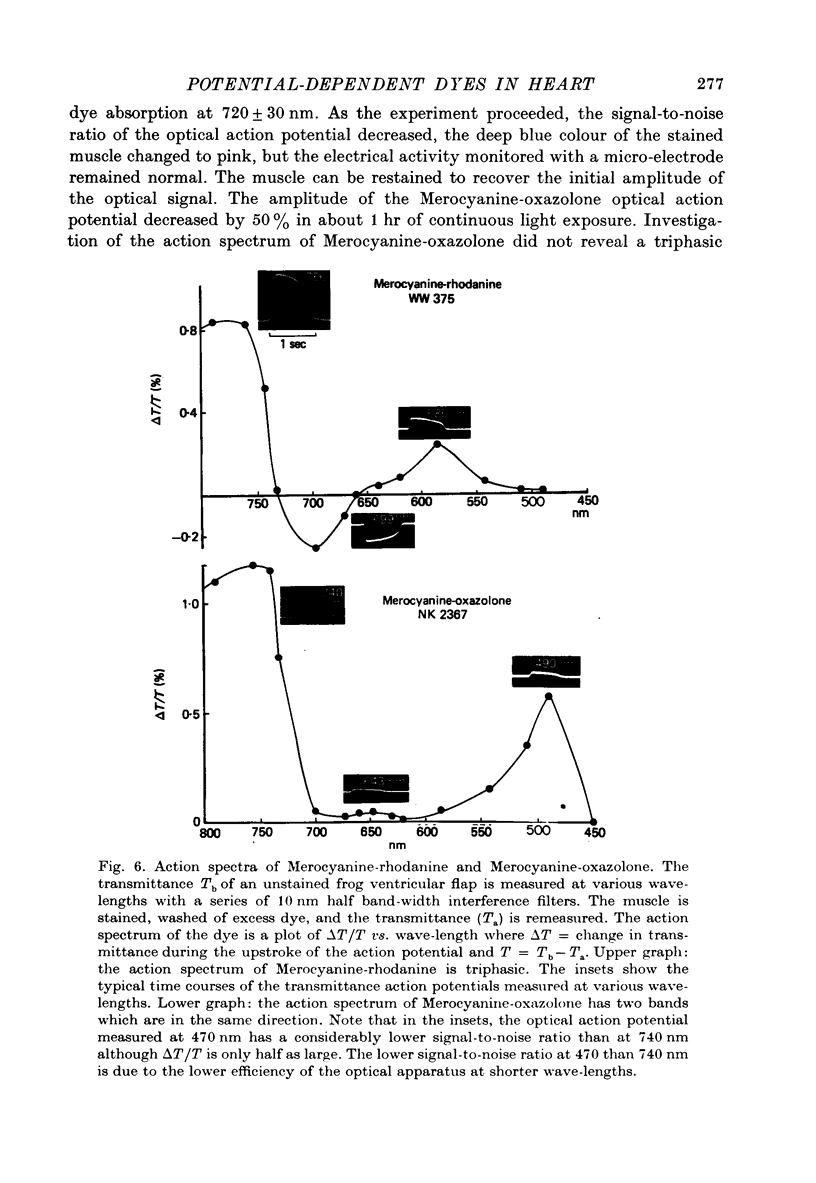
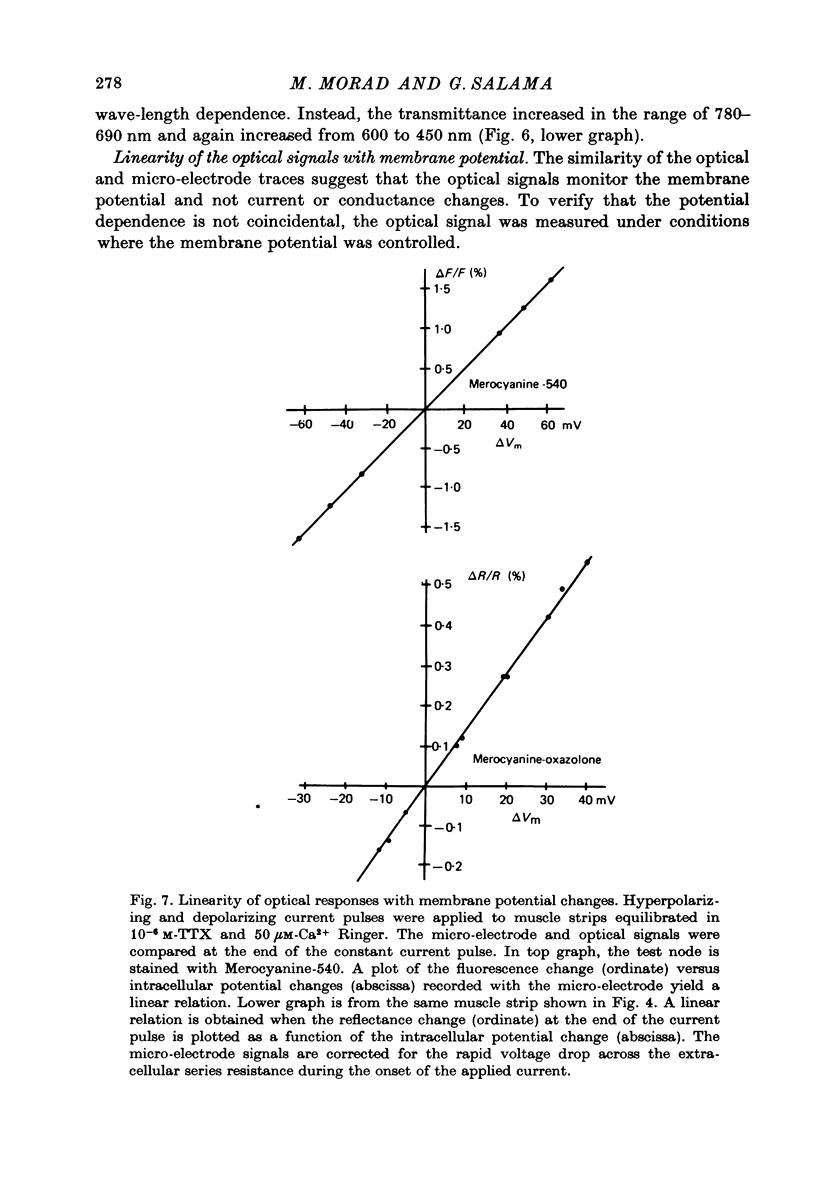
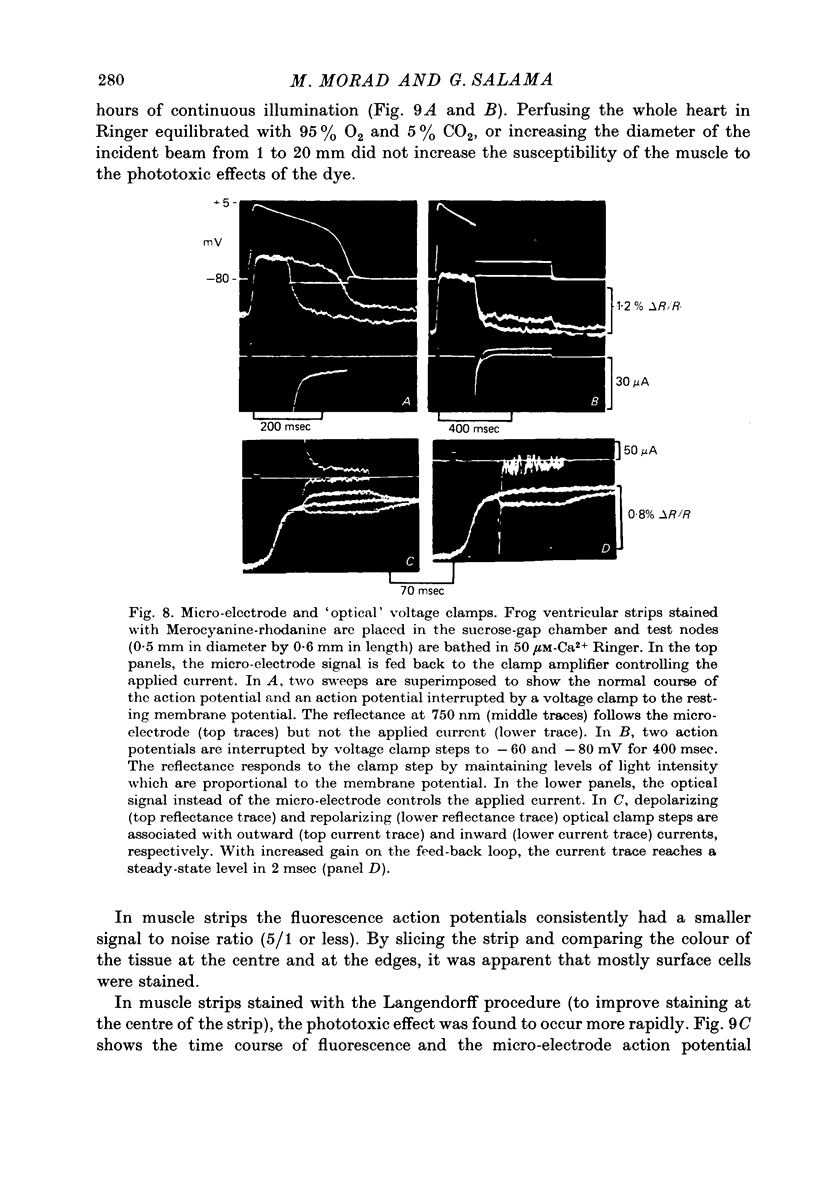
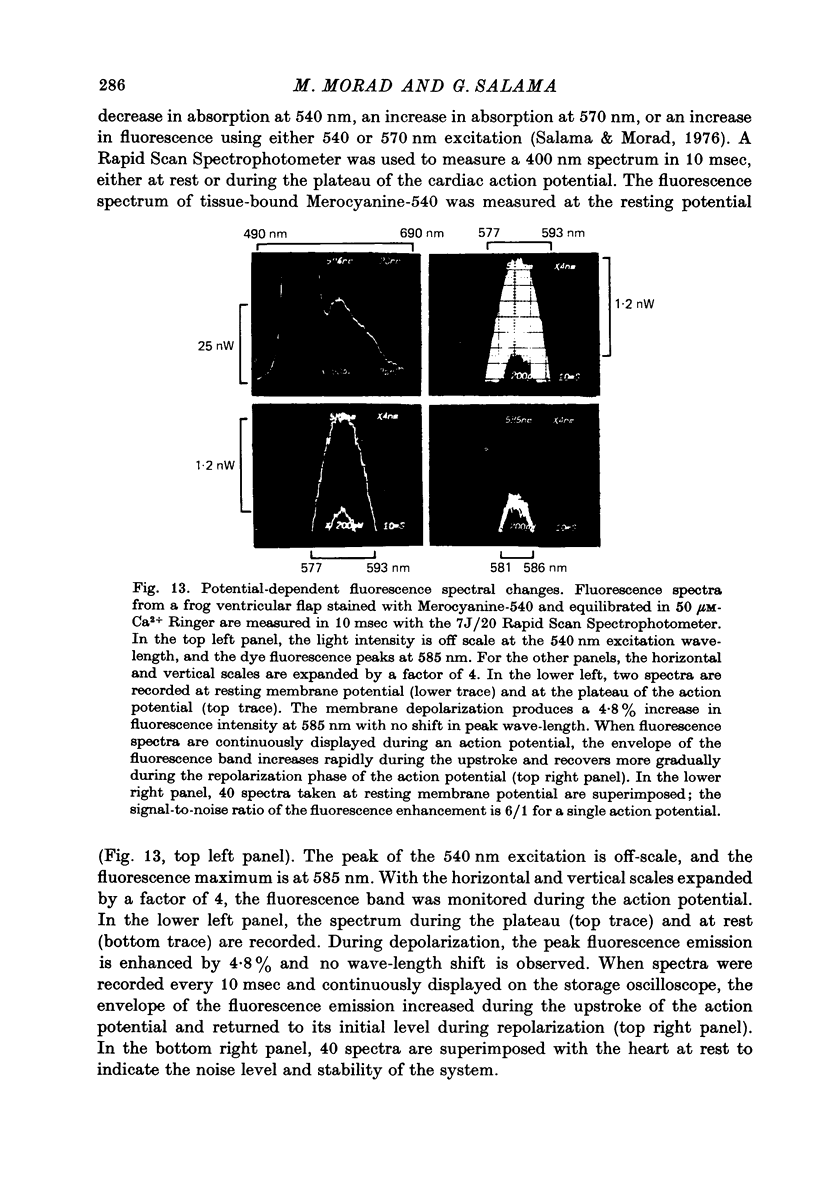
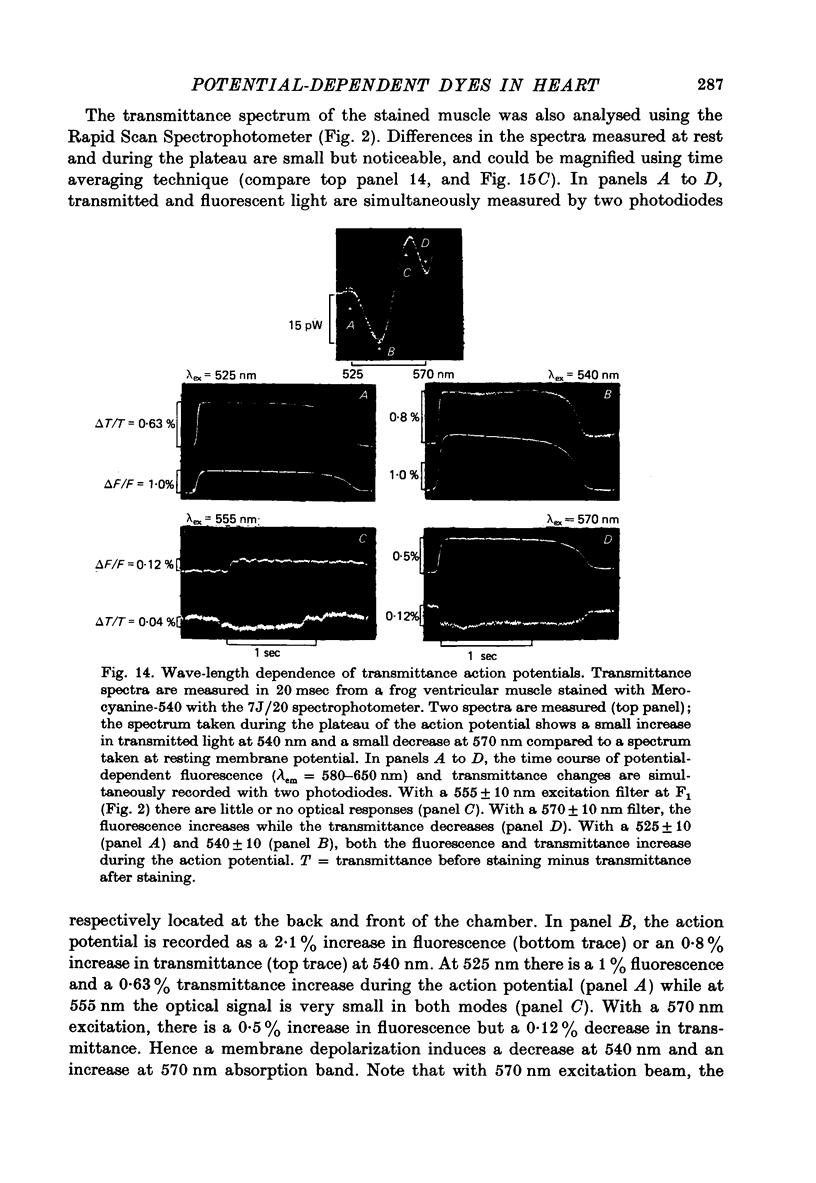
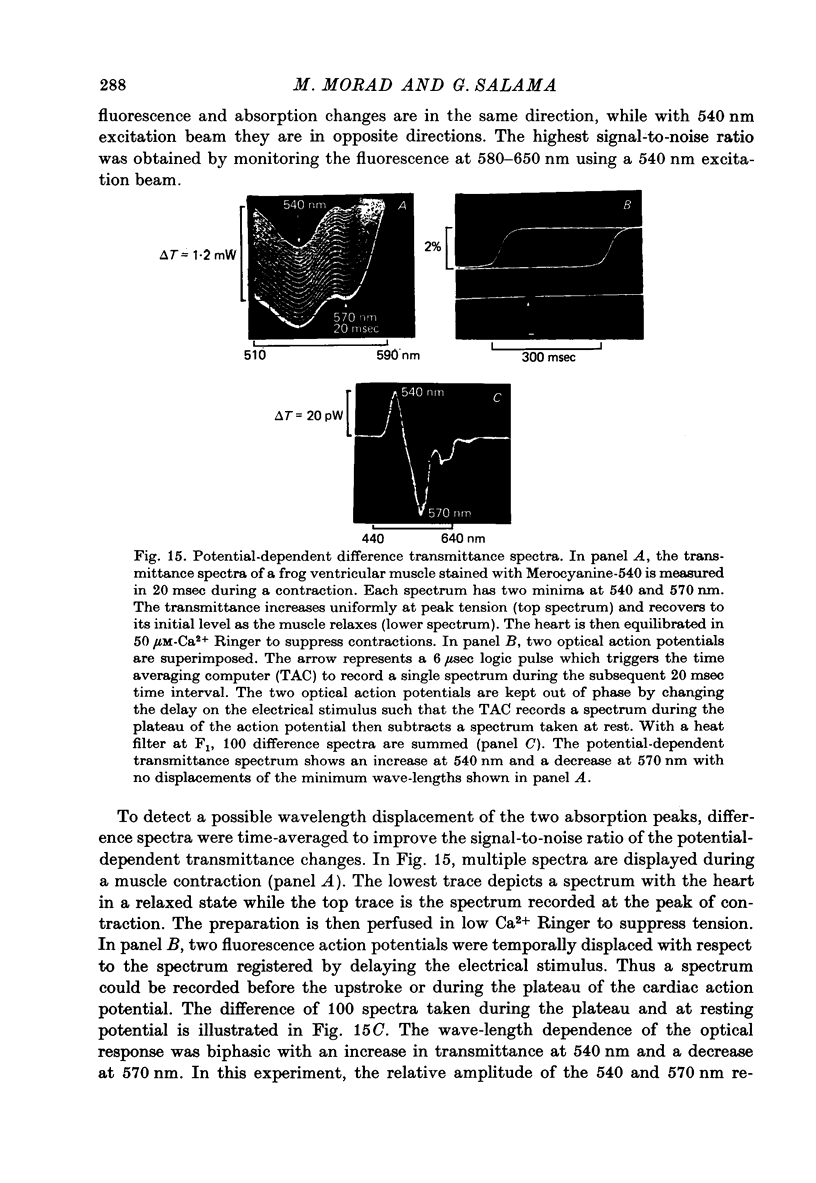
1. The fluorescent dye Merocyanine-540 and the two weakly fluoresecnet dyes Merocyanine-rhodanine and Merocyanine-oxazolone are shown to respond as optical probes of membrane potential in heart muscle. 2. In frog hearts stained with Merocyanine-540, the absorption at 540 nm decreases by 0.1-1.0% and increase at 570 nm excitation wave-length, the fluorescence increases by 1-2%. The time course of all three optical measurements follows the kinetics of the action potential. 3. Merocyanine-rhodanine exhibits potential-dependent optical responses through a 0.5% decrease in absorption at 750 nm, and Merocyanine-oxazolone has a 1.0% decrease in absorption at 720 nm. Their optical responses have a signal-to-noise ratio of 100/1 and 500/1, respectively. 4. The action spectrum of Merocyanine-rhodanine is triphasic in frog heart with an increase in transmittance from 780 to 700, a decrease from 700 to 600, and increase from 600 to 450 nm. Merocyanine-oxazolone shows only increases in transmittance during membrane depolarization. 5. The optical responses of these probes are linear with respect to changes in membrane potential. 6. Pharmacological agents or ionic interventions do not alter the membrane potential sensitivity of Merocyanine-540. 7. Rapid spectrophotometric measurements at various phases of the action potential indicate that the potential dependent optical signals of Merocyanine-540 are produced by changes in amplitude of fluorescence and absorption bands. The lack of wave-length displacement as a function of membrane potential, i.e. electrochromism, is not the mechanism governing the voltage sensitivity of Merocyanine-540. 8. The data suggest that these Merocyanine dyes bind to the plasma membrane and serve as linear optical probes of membrane potential in heart muscle.
Full text
PDF


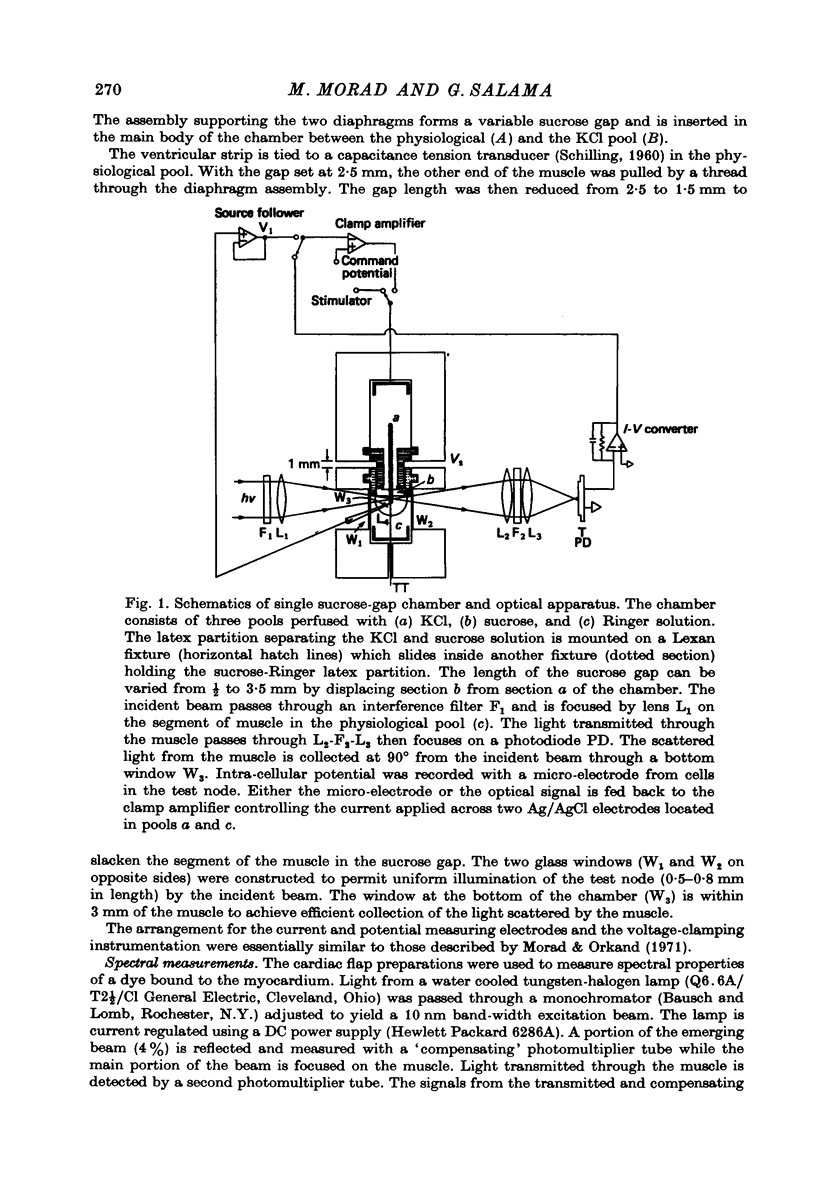
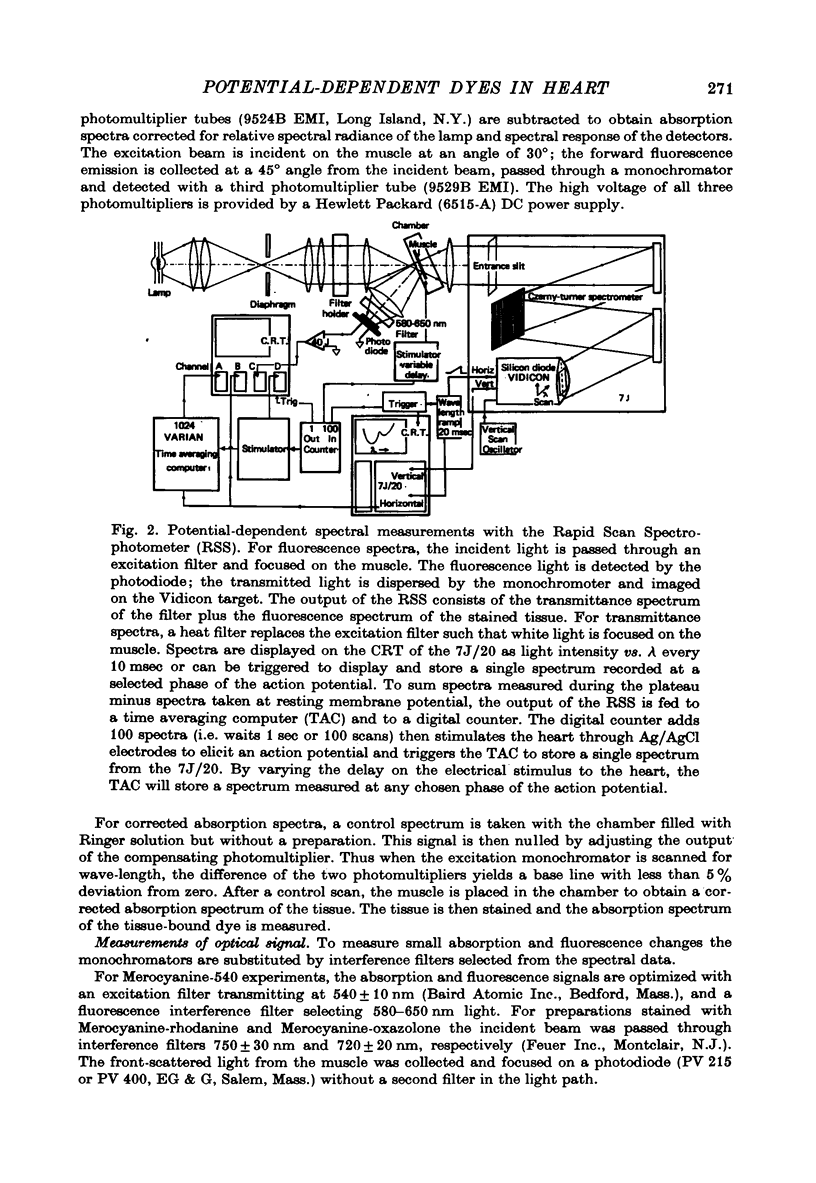


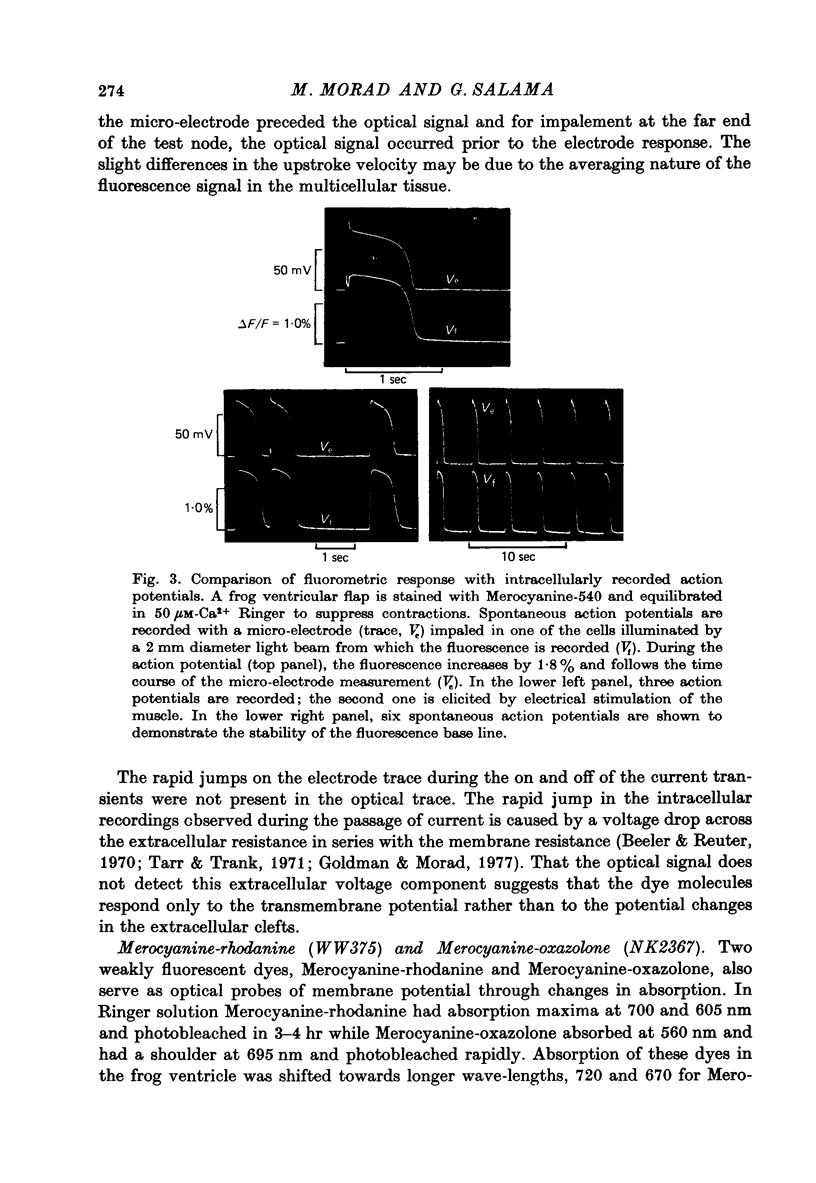
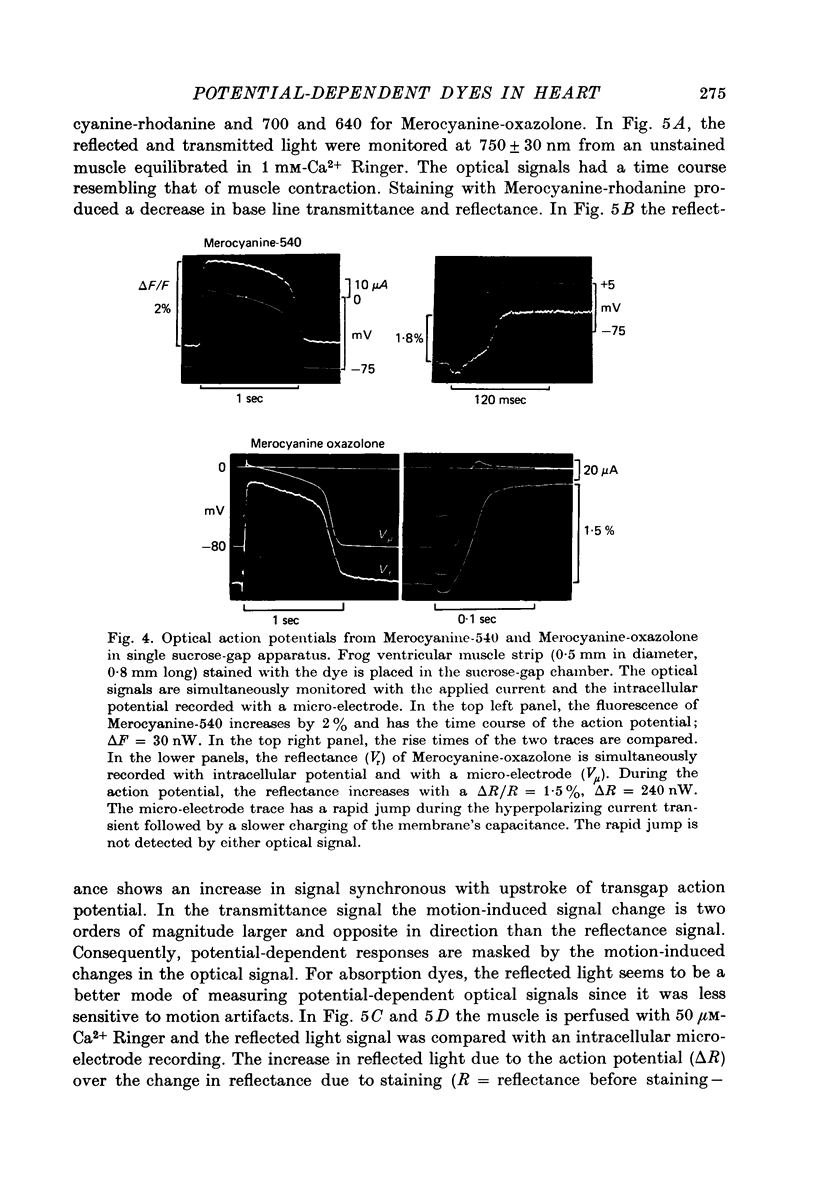









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F. Fluorescent probes in nerve membranes. Annu Rev Biophys Bioeng. 1975;4(00):287–310. doi: 10.1146/annurev.bb.04.060175.001443. [DOI] [PubMed] [Google Scholar]
- Crumly H. J., Jr, Pinder R. M., Hinshaw W. B., Goldberg L. I. Dopamine-like renal and mesenteric vasodilation caused by apomorphine 6-propylnorapomorphine and 2-amino-6, 7-dihydroxy-1,2,3,4-tetrahydronaphthalene. Nature. 1976 Feb 19;259(5544):584–587. doi: 10.1038/259584a0. [DOI] [PubMed] [Google Scholar]
- Davila H. V., Salzberg B. M., Cohen L. B., Waggoner A. S. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nat New Biol. 1973 Jan 31;241(109):159–160. doi: 10.1038/newbio241159a0. [DOI] [PubMed] [Google Scholar]
- Goldman Y., Morad M. Measurement of transmembrane potential and current in cardiac muscle: a new voltage clamp method. J Physiol. 1977 Jul;268(3):613–654. doi: 10.1113/jphysiol.1977.sp011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Gilai A., Dingeman D. Dye absorption changes in single muscle fibers: an application of an automatic balancing circuit. Pflugers Arch. 1976 Apr 6;362(3):285–287. doi: 10.1007/BF00581183. [DOI] [PubMed] [Google Scholar]
- Oetliker H., Baylor S. M., Chandler W. K. Simultaneous changes in fluorescence and optical retardation in single muscle fibres during activity. Nature. 1975 Oct 23;257(5528):693–696. doi: 10.1038/257693a0. [DOI] [PubMed] [Google Scholar]
- Page S. G., Niedergerke R. Structures of physiological interest in the frog heart ventricle. J Cell Sci. 1972 Jul;11(1):179–203. doi: 10.1242/jcs.11.1.179. [DOI] [PubMed] [Google Scholar]
- Pooler J. Photodynamic alteration of sodium currents in lobster axons. J Gen Physiol. 1972 Oct;60(4):367–387. doi: 10.1085/jgp.60.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Davila H. V. A large change in dye absorption during the action potential. Biophys J. 1974 Dec;14(12):983–986. doi: 10.1016/S0006-3495(74)85963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama G., Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976 Feb 6;191(4226):485–487. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Davila H. V., Cohen L. B. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973 Dec 21;246(5434):508–509. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Warashina A., Pant H. Studies of light emission, absorption and energy transfer in nerve membranes labelled with fluorescent probes. Biophys Chem. 1976 Jan;4(1):1–13. doi: 10.1016/0301-4622(76)80001-4. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Grinvald A. Mechanisms of rapid optical changes of potential sensitive dyes. Ann N Y Acad Sci. 1977 Dec 30;303:217–241. [PubMed] [Google Scholar]


