Abstract
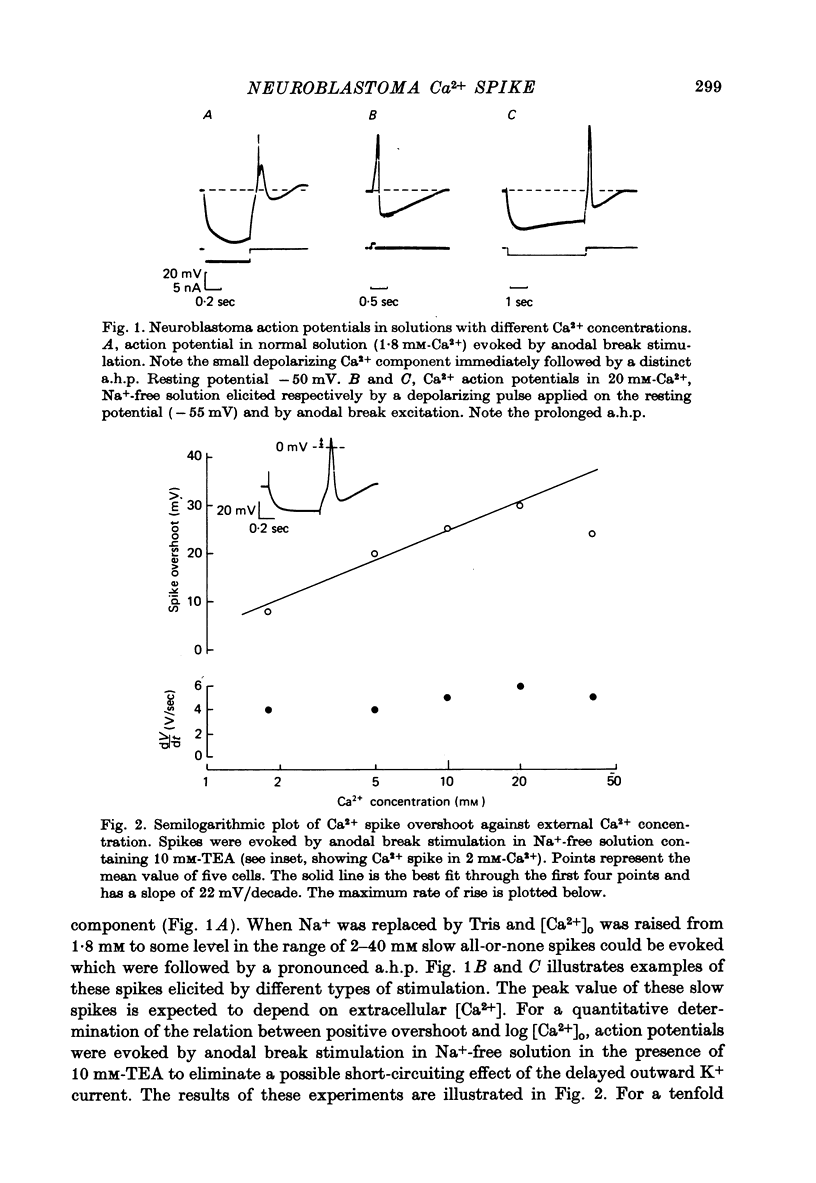
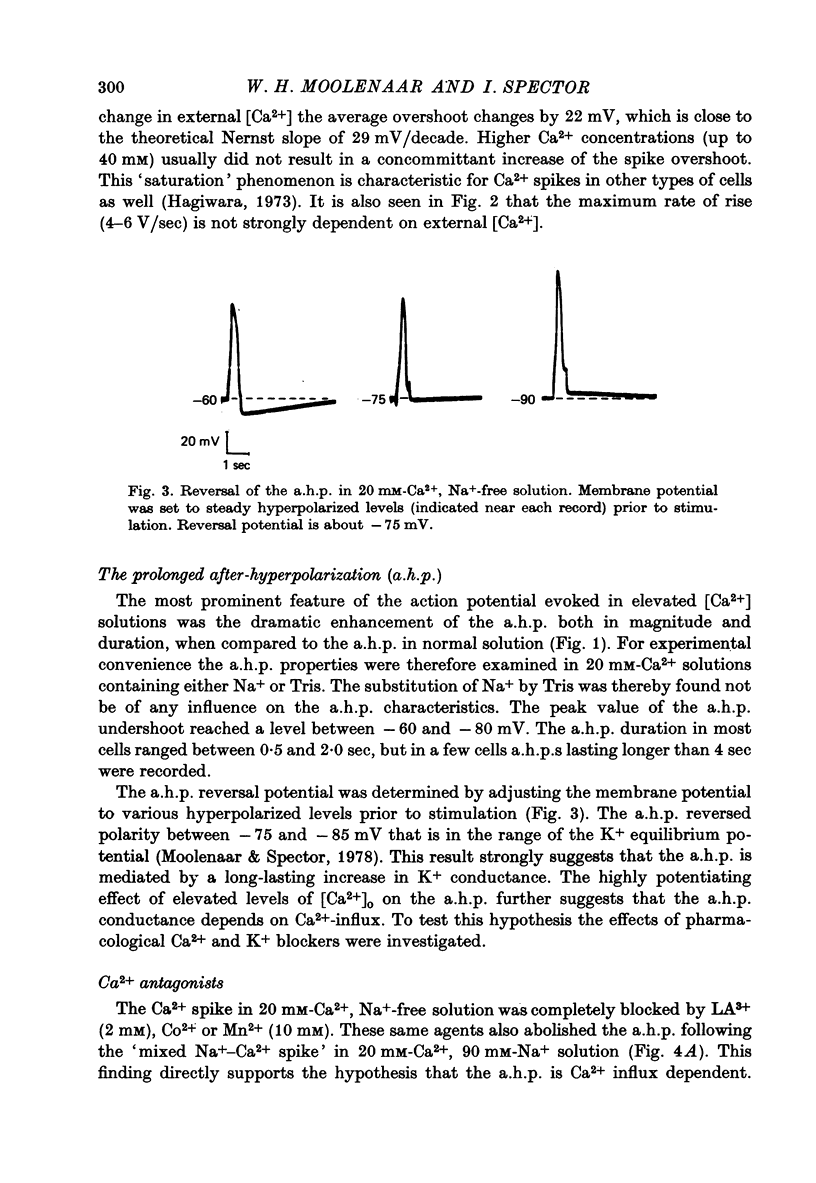
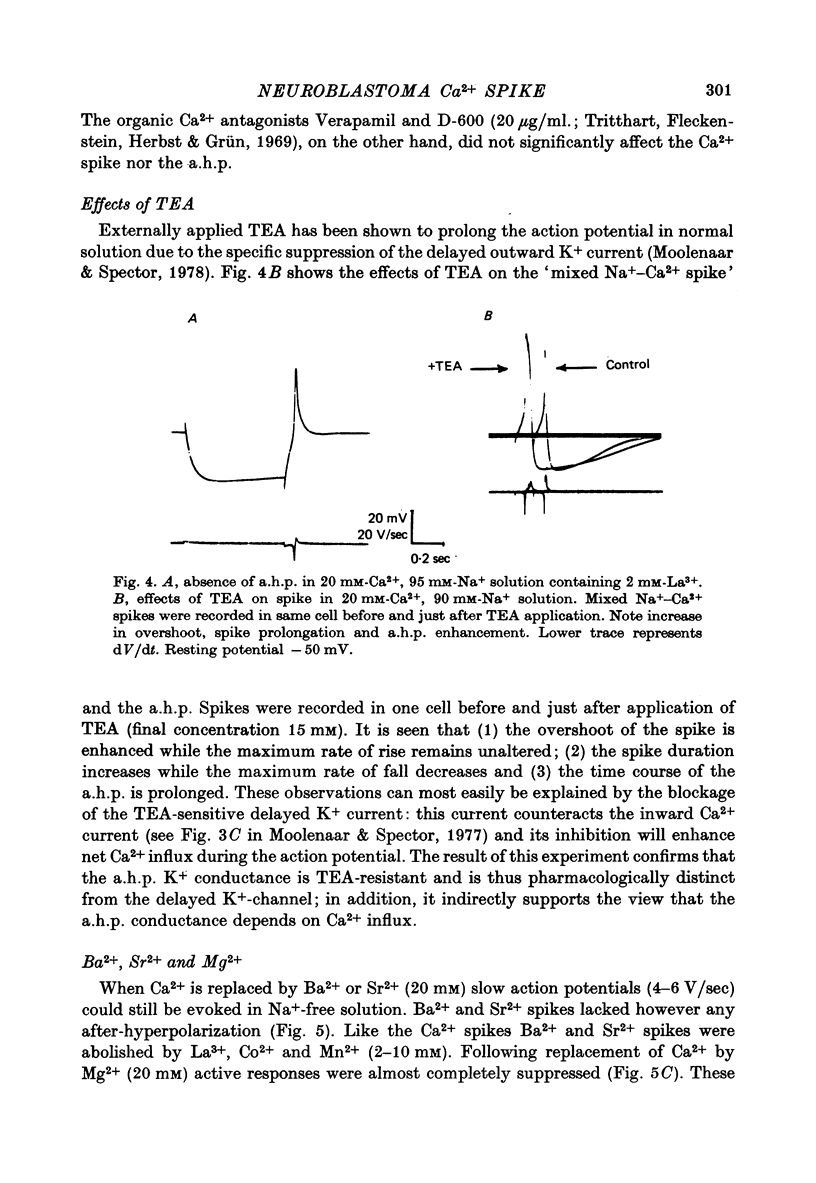
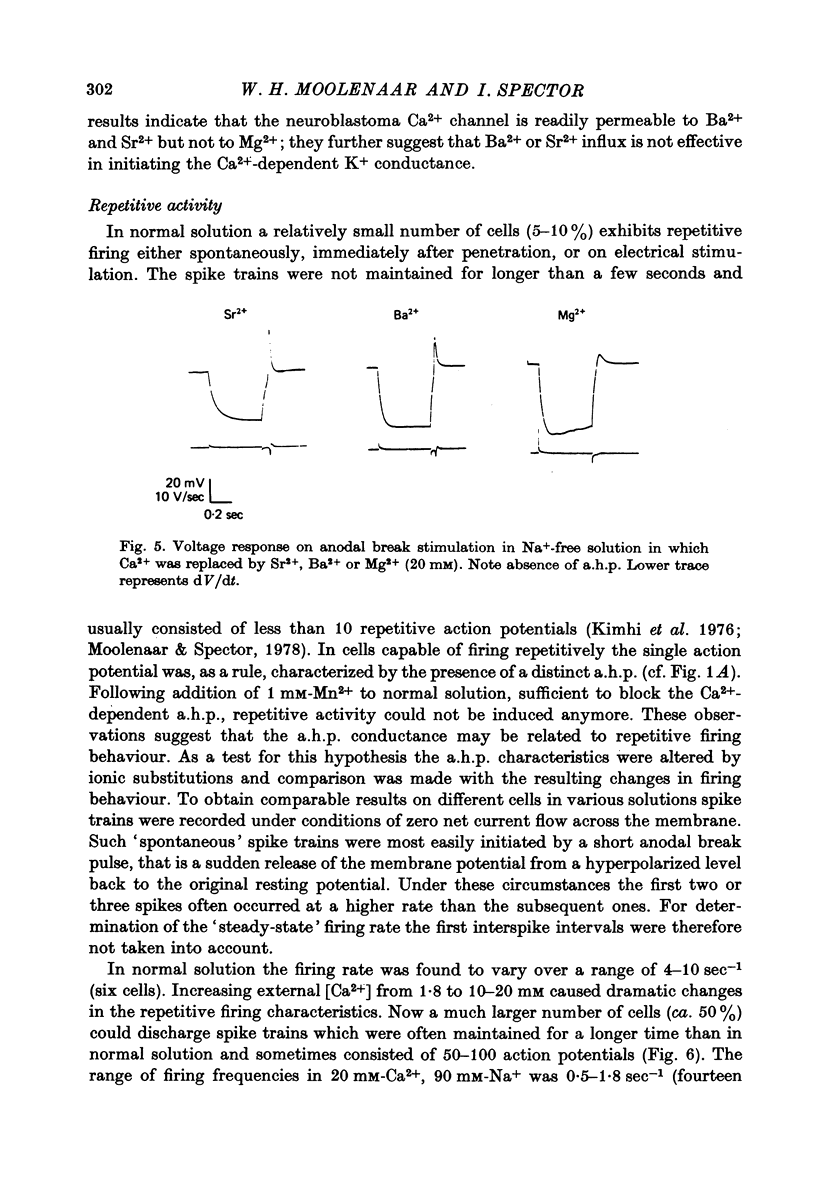
1. Action potentials elicited in solutions with elevated [Ca2+] (1.8-40 mM) have been studied in differentiated cells of mouse neuroblastoma clone N1E-115 in tissue culture. 2. The action potential in high [Ca2+] solutions containing eithr Na+ or Tris is followed by a prolonged after-hyperpolarization (a.h.p.) lasting 0.5-4 sec. The a.h.p. reverses sign between -75 and -85 mV. 3. Externally applied tetraethylammonium (TEA, 15 mM) increases the Ca2+ spike overshoot, prolongs the falling phase and enhances the a.h.p. duration. The a.h.p. is inhibited by Ca2+ antagonists such as La3+, Co2+ and Mn2+. 4. After replacement of Ca2+ by Ba+ or Sr2+ (20mM) action potentials can still be elicited in Na+-free solution, but no a.h.p. is observed. 5. Increasing [Ca2+] from 1.8 up to 20 mM results in an increased capability of neuroblastoma cells to fire repetitively and in a consistent reduction of the firing rate from about 4-10 sec-1 to 0.5-1.8 sec-1. 6. It is concluded that Ca2+ entry during the action potential activates a TEA-resistant K+ conductance which gives rise to the prolonged a.h.p. Data from repetitively firing cells are consistent with the view that the a.h.p. plays a role in the regulation of low-frequency firing.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldissera F., Gustafsson B. Regulation of repetitive firing in motoneurones by the afterhyperpolarization conductance. Brain Res. 1971 Jul 23;30(2):431–434. doi: 10.1016/0006-8993(71)90096-5. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busis N. A., Weight F. F. Spike after-hyperpolarisation of a sympathetic neurone is calcium sensitive and is potentiated by theophylline. Nature. 1976 Sep 30;263(5576):434–436. doi: 10.1038/263434a0. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Linström S., Zangger P. Firing behaviour of dorsal spinocerebellar tract neurones. J Physiol. 1978 Feb;275:321–343. doi: 10.1113/jphysiol.1978.sp012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhi Y., Palfrey C., Spector I., Barak Y., Littauer U. Z. Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc Natl Acad Sci U S A. 1976 Feb;73(2):462–466. doi: 10.1073/pnas.73.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. EGTA and motoneuronal after-potentials. J Physiol. 1978 Feb;275:199–223. doi: 10.1113/jphysiol.1978.sp012186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Membrane currents examined under voltage clamp in cultured neuroblastoma cells. Science. 1977 Apr 15;196(4287):331–333. doi: 10.1126/science.557842. [DOI] [PubMed] [Google Scholar]
- Peacock J. H., Nelson P. G., Goldstone M. W. Electrophysiologic study of cultured neurons dissociated from spinal cords and dorsal root ganglia of fetal mice. Dev Biol. 1973 Jan;30(1):137–152. doi: 10.1016/0012-1606(73)90053-5. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Calvin W. H. Nature of conductances underlying rhythmic firing in cat spinal motoneurons. J Neurophysiol. 1973 Nov;36(6):955–973. doi: 10.1152/jn.1973.36.6.955. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]


