Abstract
To investigate the possible effects of glycoinositolphospholipid (GIPL) from Trypanosoma cruzi on human antigen presenting cells, we tested their effects on lipopolysaccharide (LPS)-stimulated human macrophages and dendritic cells (DC). Human macrophages or DC were incubated with GIPL (50 μg/ml) and LPS (500 pg/ml) and tumor necrosis factor alpha (TNF-α), interleukin 8 (IL-8), IL-10, and IL-12p40 levels in supernatants were analyzed by enzyme-linked immunosorbent assay. TNF-α, IL-10, and IL-12 secretion were significantly decreased by GIPL both in macrophages and DC. In contrast, GIPL did not alter IL-8 production. We also analyzed the expression of CD80, CD86, HLA-DR, CD40, and CD57 on the macrophage surface after stimulation with LPS in the presence or absence of T. cruzi GIPL. GIPL led to a down-regulation in the expression of all tested molecules. We additionally examined the influence of T. cruzi GIPL on the response of human DC to LPS. LPS-induced HLA-DR, CD83, and CD86 up-regulation was significantly inhibited by GIPL. A slight down-regulation in CD80 and CD40 expression on DC surfaces in the presence of GIPL was also noticed. Similarly, GIPL led to down-modulation of CD83, CD80, CD86, and HLA-DR surface expression and TNF-α and IL-10 production when DC were stimulated by CD40L. The ceramide portion of GIPL was responsible for most of the activity exhibited by the whole molecule. Considering the important role of the immune response in determining the fate of the host-parasite relationship, the immunoregulatory activities of T. cruzi GIPL are potentially important for parasite evasion and then pathogenesis of infection with protozoan parasites.
Glycosylphosphatidylinositol (GPI) family molecules from protozoan parasites play important roles in the establishment of several parasitic infections. GPI molecules from Plasmodium sp. activate signal transduction pathways in the host immune system, resulting in secretion of TNF-α (28). Lipophosphoglycan (LPG) from Leishmania sp. is able to deactivate human monocytes and block their ability to undergo a respiratory burst (11, 20). The most-abundant cell surface molecules on the epimastigote and metacyclic forms of Trypanosoma cruzi are a small family of type 1 glycoinositolphospholipids (GIPLs) (7, 9, 10, 22) and a family of GPI-anchored heavily O-glycosylated mucin-like glycoproteins (23, 27-29). GIPLs from T. cruzi down-regulate murine T-cell activation through their ceramide domain (16), while augmenting B-cell activation and immunoglobulin (Ig) secretion through their glycan chain (4). On the other hand, GPI-anchored mucin-like glycoproteins isolated from T. cruzi induce interleukin 12 (IL-12) and tumor necrosis factor alpha (TNF-α) synthesis by murine macrophages (5, 14). The GPI moiety of the mucin-like glycoproteins is sufficient to trigger proinflammatory cytokine production (14). Therefore, parasite-derived GPI molecules can induce both activating and deactivating signal-transducing effects on the murine immune system, contributing to parasite pathogenicity. Nothing is known of the effect of T. cruzi GIPL on the human innate immune system.
Dendritic cells (DC) are of utmost importance in initiating primary immune responses (2) as they produce IL-12, which plays a key role in the induction of cell-mediated immunity to intracellular pathogens by triggering the production of gamma interferon from NK and T cells (24). The entry of T. cruzi in macrophages also leads to different molecular interactions that induce the innate immune response (1), including IL-12 and TNF-α production (31). The induction of IL-12 early in T. cruzi infection initiates murine innate resistance, which is dependent on gamma interferon and TNF-α (1, 17) and ensures the induction of an efficient adaptive host response.
In the present report we analyzed the effects of GIPLs from T. cruzi on the very early responses of human macrophages or DC. Understanding how parasite molecules interfere with LPS-induced costimulatory surface molecules and cytokine production by these cells may help in elucidating T. cruzi survival mechanisms in the human system.
MATERIALS AND METHODS
Human macrophages.
Peripheral blood mononuclear cells (PBMC) from healthy volunteers' buffy coats (Hemocentro do Estado Da Bahia) were obtained through passage over Ficoll-Hypaque gradient (Sigma-Aldrich, St. Louis, Mo.). The cells were then washed and resuspended in RPMI 1640 medium (Gibco, Rockville, Md.) supplemented with 2 mM l-glutamine, penicillin (100 U/ml), gentamicin (100 μg/ml), and 10% heat-inactivated AB human serum (Sigma-Aldrich), termed complete medium, at a concentration of 5 × 106 cells/ml. These cells were placed on 24-well plates (Costar, Corning Incorporated, Corning, N.Y.), at 1 ml/well, and incubated for 2 h at 37°C in 5% CO2. Nonadherent cells were removed from the plates, and adherent cells were cultured in complete medium for 7 days. Either the cells were pretreated with T. cruzi GIPL (50 μg/ml) for 3 h and then incubated with LPS (500 pg/ml) for 48 h, or GIPL and LPS were simultaneously added to macrophages, as indicated in Results. Supernatants were harvested and kept at −20°C until tested for cytokine content by enzyme-linked immunosorbent assay (ELISA).
Human DC.
Human DC were generated from PBMC as previously described (26). Briefly, PBMC were separated on multistep Percoll gradients (Amersham Pharmacia, São Paulo, Brazil) and the light density fraction from the 40 to 50% interface was recovered and depleted of CD19+ and CD2+ cells using magnetic beads coated with specific antibodies (MicroBeads; Miltenyi Biotech, Inc, Sunnyvale, Calif.). The remaining cells were cultured at 3 × 105/ml in RPMI 1640 medium plus 10% fetal calf serum (FCS) supplemented with granulocyte-macrophage colony-stimulating factor (50 ng/ml) and IL-4 (1,000 U/ml) at 37°C at 5% CO2 in air. DC were used after 8 days of culture. The cells were characterized by flow cytometry (FACSort; Becton Dickinson, San Diego, Calif.), using antibodies anti-CD1a, CD14, CD80, CD86, CD40, CD54, HLA-DR, and CD83 conjugated to phycoerythrin (PE) or fluorescein isothiocyanate (FITC), as well as isotype controls. These cells were stimulated with LPS (500 pg/ml) or CD40L (Ancell Corporation, Bayport, Minn.) at a concentration of 1 μg/ml.
Isolation and purification of trypanosomatid GIPLs and their lipid components.
The procedure for isolation and purification of trypanosomatid GIPLs and their lipid components was the same as described previously (22). Briefly, epimastigotes of T. cruzi (G or Y strains) were grown in brain heart infusion-hemin medium supplemented with 5% FCS. At early stationary growing phase (5 days at 26°C, with shaking), cells were harvested, washed three times with 0.9% NaCl, and stored frozen. Cells were thawed and extracted three times with cold water, and the pellet was recovered by centrifugation (7,000 × g, 10 min). The remaining cell debris was extracted with 45% aqueous phenol (vol/vol) at 75°C for 15 min. The aqueous layer from phenol extraction was dialyzed, freeze-dried, dissolved in water, and applied to a column of Bi-Gel P-100 (2 by 60cm). The excluded material was lyophilized, and the GIPLs were extracted by chloroform-methanol-water (10:10:3, vol/vol/vol). The purified GIPL could be dissolved in water and sterile filtered for use in tissue culture. The GIPL appeared on sodium dodecyl sulfate-polyacrylamide gel electrophoresis as a fast-moving, single molecular species. Virtual absence of contaminating peptidic material was confirmed by absence of peptide-derived signals in nuclear magnetic resonance analysis of the purified material (7). For isolation of the lipid moiety, the intact GIPL was subjected to alkaline hydrolysis (1 M KOH, for 48 h at 37°C) (30). After neutralization with acetic acid, nonpolar material from G strain GIPL, comprising a ceramide, was obtained by chloroform extraction, purified by silica chromatography, and evaporated to dryness. The ceramide was dissolved in phosphate-buffered saline (PBS) containing 10% ethanol, by heating at 60 to 90°C, to a stock concentration of 500 μg/ml. The ceramide domain was characterized as N-lignoceroyl-sphinganine by gas chromatography-mass spectrometry (7, 22). Unless otherwise indicated, results refer to the use of the Y strain GIPL.
Flow cytometry.
Cells (5 × 105 cells/sample) were resuspended in PAB (PBS, 1% bovine serum albumin, and 0.05% sodium azide) and blocked with 10% FCS and Fc block (20 μg/ml of mouse Ig) for 30 min on ice. Cells were then labeled with monoclonal antibodies or respective controls for an additional 30 min. Cells were fixed with 1% paraformaldehyde in PBS and analyzed on a Becton Dickinson flow cytometer (FACSort). We used the following antibodies in these experiments: PE-labeled anti-human CD1a (clone HI 149, mouse IgG1); PE- or FITC-labeled anti-human CD80 (clone L307.4, mouse IgG1); PE- or FITC-labeled anti-human CD86 (clone 2331, FUN-1 mouse IgG1); Cyanine-labeled anti-human CD40 (clone 5C-3, mouse IgG1); PE-labeled anti-human CD83 (clone HB15e, mouse IgG1); FITC-labeled anti-human HLA DR, DP, and DQ (clone TU 39, mouse IgG2a); PE-, FITC-, or Cy-labeled mouse IgG1, κ chain (clone MOPC-21, isotype control); and FITC-labeled mouse IgG2a, κ chain (clone MOPC-173, isotype control).
Cytokine assays.
Levels of TNF-α, IL-12, IL-10, IL-8, and IL-6 in supernatants were determined by ELISA using commercial anticytokine antibody pairs (Pharmingen, San Diego, Calif.) according to the manufacturer's protocol. Human recombinant TNF-α, IL-12, IL-10, IL-8, and IL-6 (Pharmingen) were used to generate standard curves.
Statistical analysis.
Comparison of cytokine levels in the presence or absence of T. cruzi GIPL was done by nonparametric Wilcoxon test. All tests were performed using Prism software (GraphPad Software, Inc., San Diego, Calif.). Differences were considered significant at P < 0.05.
RESULTS
GIPLs inhibit LPS-induced cytokine production by human macrophages.
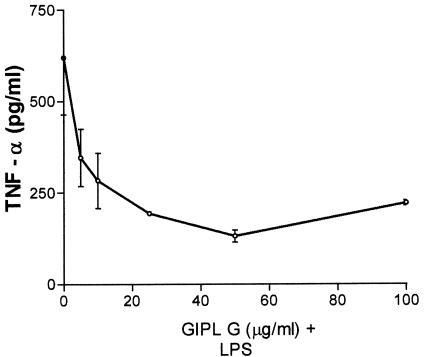
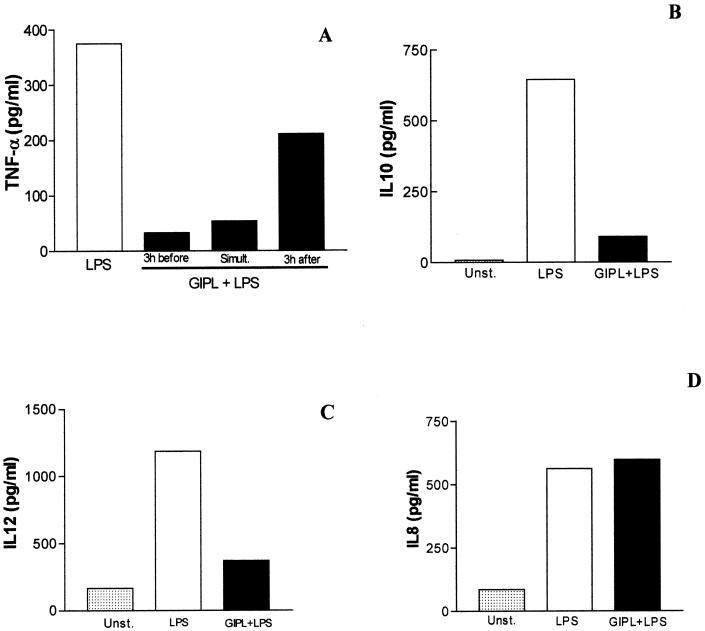
To investigate the possible effects of trypanosome GIPLs on APCs, we tested their effects on LPS-stimulated human macrophages. Macrophages obtained from PBMC were incubated with increasing concentrations of GIPL (5 to 100 μg/ml) and LPS (500 pg/ml) for 48 h at 37°C at 5% CO2. After this period the supernatants were harvested and the TNF-α content was analyzed by ELISA. Increasing the doses of GIPL from the Y or G strain caused a significant reduction in LPS-induced TNF-α levels (Fig. 1). This significant reduction in TNF-α levels was only observed when T. cruzi GIPL was incubated 3 h before LPS addition or when GIPL was simultaneously added to LPS, whereas the addition of GIPL 3 h after LPS stimulation did not induce significant reduction in the levels of TNF-α (Fig. 2A).
FIG. 1.
Effect of G strain T. cruzi GIPL on LPS-stimulated human macrophages. Macrophages obtained from healthy volunteers' PBMC were incubated with increasing doses of GIPL (5 to 100 μg/ml) simultaneously with LPS (500 pg/ml). The data shown are from a single experiment, representative of three separate experiments. Error bars, standard deviations.
FIG. 2.
Effect of T. cruzi GIPL on LPS-stimulated macrophages. (A) Macrophages were incubated with Y strain GIPL (50 μg/ml) for 3 h before or after LPS addition or simultaneously added to LPS (500 pg/ml). (B, C, and D) Macrophages obtained from PBMC were kept unstimulated (Unst.), incubated with LPS alone (500 pg/ml), or incubated with GIPL (50 μg/ml) plus LPS (500 pg/ml) for 48 h. After this period, supernatants were harvested and their contents were analyzed by ELISA for determination of the production of IL-10 (B), IL-12p40 (C), and IL-8 (D). The data shown are from a single experiment, representative of five separate experiments.
We next investigated whether T. cruzi GIPL would affect the production of other cytokines by LPS-stimulated human macrophages. Macrophages were incubated with GIPL (50 μg/ml) and LPS (500 pg/ml) for 48 h, and concentrations of IL-12, IL-10, and IL-8 were determined by ELISA. Both IL-10 and IL-12p40 secretion were significantly decreased after incubation with GIPL (Fig. 2B and C). By contrast, GIPL did not alter IL-8 production (Fig. 2D). GIPL is, then, able to impair the production of proinflammatory cytokines, which are important to the innate immune response.
Effects of T. cruzi GIPLs on expression of costimulatory molecules on the macrophage surface.
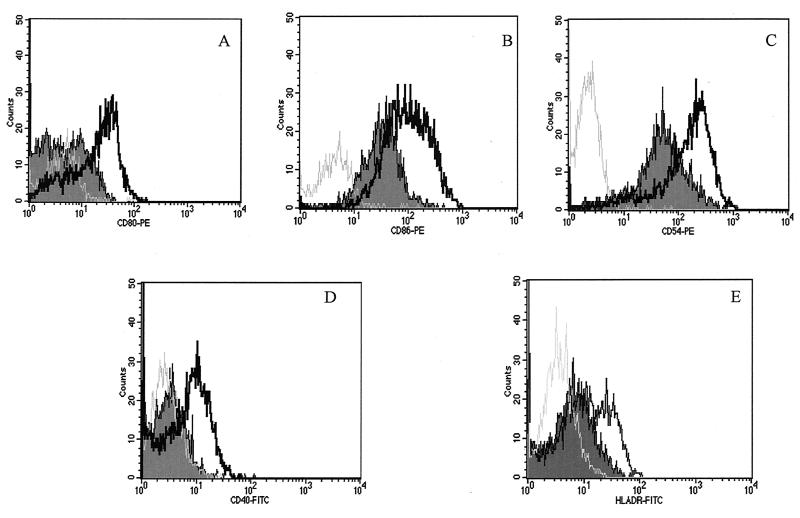
Since costimulatory molecules play an essential role in the activation and maintenance of T-cell responses, we analyzed the expression of CD80, CD86, CD54, CD40, and HLA-DR on macrophage surface after stimulation with LPS, in the presence or absence of T. cruzi GIPL. GIPL led to a down-regulation in the expression of all these molecules on LPS-induced macrophages (Fig. 3). GIPL seems to contribute to the impairment of the acquired immune response.
FIG. 3.
Effect of T. cruzi GIPL on costimulatory expression on LPS-stimulated macrophage surfaces. Macrophages obtained from PBMC were incubated in the presence or absence of GIPL (50 μg/ml) plus LPS (500 pg/ml) for 48 h. After this period, the cells were harvested and labeled with specific antibodies to be analyzed by flow cytometry. (A) CD80 expression; (B) CD86 expression; (C) CD54 expression; (D) CD40 expression; (E) HLA-DR expression. The thick black line represents the costimulatory molecule expression from cells stimulated with LPS, the curve that is shaded represents the expression of the costimulatory molecule from cells treated with LPS in the presence of GIPL (Y strain [50 μg/ml]), and the gray line represents cells treated with LPS and stained with isotype antibody controls. The data shown are from a single experiment, representative of five separate experiments.
Effects of T. cruzi GIPL on cytokine production by human DC.
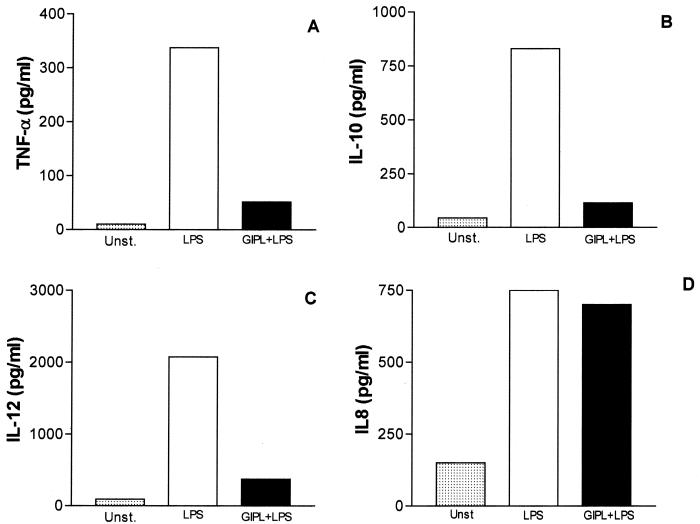
We also investigated whether T. cruzi GIPL would affect LPS-induced cytokine production by human DC. Similarly to the effect of GIPL on macrophages, IL-12p40, IL-10, and TNF-α production, but not IL-8 production, were significantly decreased after treatment of cells with GIPL (Fig. 4). This effect could contribute to parasite protection from the innate responses, permitting the establishment of infection.
FIG. 4.
Effect of T. cruzi GIPL on LPS-stimulated DC. DC obtained from PBMC from healthy volunteers (see Material and Methods) were kept unstimulated (Unst.), incubated with LPS alone (500 pg/ml), or incubated with GIPL (50 μg/ml) plus LPS (500 pg/ml) for 48 h. After this period, supernatants were harvested and their contents were analyzed by ELISA for determination of the production of TNF-α (A), IL-10 (B), IL-12p40 (C), and IL-8 (D). The data shown are from a single experiment, representative of five separate experiments.
Effect of GIPL on DC maturation induced by LPS stimulation.
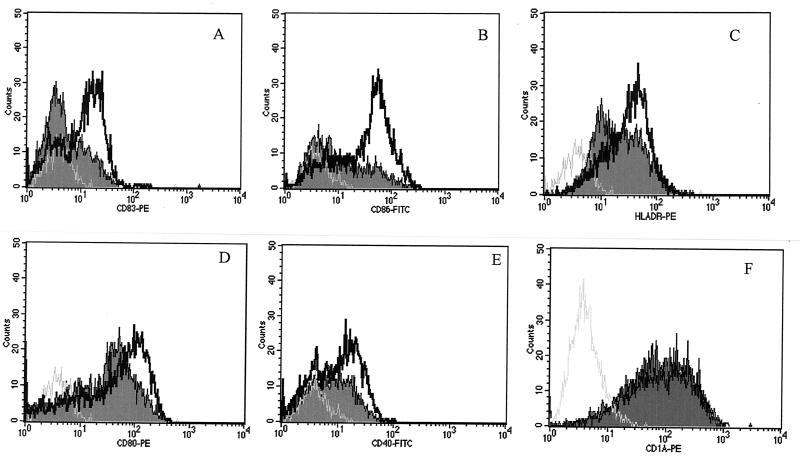
Since DC maturation is an essential process during which DC acquire optimal immunostimulatory properties, we examined the influence of T. cruzi GIPL on the response of DC to LPS, which has been described to be a major stimulus for up-regulation of surface molecule expression (34). After treatment with GIPL (50 μg/ml) and simulation with LPS (500 pg/ml), DC were harvested and labeled with specific antibodies to be analyzed by flow cytometry. LPS-induced CD83, CD86, and HLA-DR up-regulation was significantly inhibited by GIPL, whereas CD80 and CD40 expression was slightly inhibited by the presence of GIPL (Fig. 5A to E). No alteration was observed on CD1a expression on the DC surface (Fig. 5F). Since the expression of CD83, CD40, and HLA-DR is associated with TNF-α and IL-12 secretion, we investigated whether the GIPL-induced decrease of the expression of these molecules was dependent on the inhibition of TNF-α production. DC treated with recombinant human TNF-α induced an increase in costimulatory expression, and addition of GIPL to these cultures did not lead to down-regulation on costimulatory expression (data not shown). Therefore, GIPL acts in an inhibitory way on DC maturation, through impairment of TNF-α production, delaying an effective immune response against T. cruzi. We also tested the ability of GIPL in inhibiting CD40L. GIPL inhibited CD40L-induced DC maturation by leading to a down-regulation on CD83, CDD86, and HLA-DR surface expression (Table 1). The presence of GIPL during DC maturation induced by CD40L also caused a decrease in TNF-α and IL-10 production (Table 1)
FIG. 5.
Effect of T. cruzi GIPL on costimulatory expression on LPS-stimulated DC surfaces. DC obtained from PBMC were incubated in the presence or absence of GIPL (50 μg/ml) plus LPS (500 pg/ml) for 48 h. After this period, the cells were harvested and labeled with specific antibodies to be analyzed by flow cytometry. (A) CD83 expression; (B) CD86 expression; (C) HLA-DR expression; (D) CD80 expression; (E) CD40 expression; (F) CD1a expression. The black line represents the costimulatory molecule expression from cells stimulated with LPS, the curve that is shaded represents the expression of the costimulatory molecule expression from cells treated with LPS in the presence of GIPL (Y strain [50 μg/ml]), and the gray line represents cells treated with LPS and stained with isotype antibody controls. The data shown are from a single experiment, representative of five separate experiments.
TABLE 1.
Effect of GIPL from T. cruzi on expression of costimulatory molecules and cytokines on human DC
| Molecule expressed or produced | % inhibitiona after treatment with:
|
|
|---|---|---|
| LPS GIPL | CD40L GIPL | |
| CD80 | 27 | 22.7 |
| CD86 | 44 | 10 |
| CD83 | 40 | 38 |
| HLA-DR | 23 | 21 |
| TNF-α | 82.2 | 50 |
| IL-10 | 26.3 | 35 |
Inhibition of expression or production in relation to cultures without GIPL.
APC-inhibitory activity of T. cruzi GIPL is due to the ceramide domain.
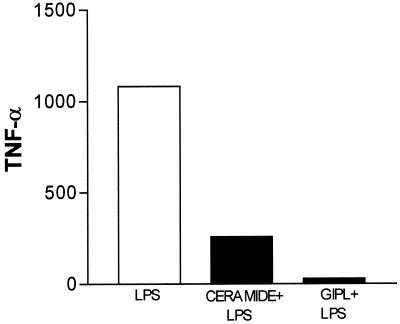
GIPLs are amphiphilic chemical structures composed of distinct oligosaccharide and lipid domains. Because of the potentially diverse biologic properties of these two domains, it was of interest to determine the APC-suppressive region of T. cruzi G strain GIPL. Isolated ceramide (12.5 to 50 μg/ml) blocked, in a dose-dependent fashion, LPS (500 pg/ml)-induced TNF-α secretion from both human macrophages and DC. Figure 6 illustrates the effects of a 12.5-g/ml concentration of ceramide and a 50-μg/ml concentration of GIPL on LPS-stimulated human macrophages. Approximately fourfold-lower concentrations of ceramide were sufficient to induce a suppressive activity similar to that mediated by intact T. cruzi GIPL in human APCs. A 4:1 relation of ceramide glycan is found in the GIPL molecule, suggesting that the ceramide domain accounts for the whole APC-suppressive activity present in the intact GIPL.
FIG. 6.
Ceramide moiety from T. cruzi GIPL (G strain) inhibited LPS-induced TNF-α secretion from macrophages. Macrophages obtained from PBMC were incubated with LPS alone or with GIPL (50 μg/ml) or isolated ceramide (12.5 μg/ml) plus LPS (500 pg/ml) for 48 h. After this period, supernatants were harvested and their contents were analyzed by ELISA determination of the production of TNF-α. The data shown are from a single experiment, representative of five separate experiments.
DISCUSSION
The present report shows that GIPL purified from T. cruzi surface exerts a suppressive activity on LPS-stimulated human macrophages or DC, inhibiting the secretion of proinflammatory cytokines like TNF-α and IL-12. GIPL also induced a down-regulation of LPS-induced costimulatory molecules on the APC surface. An inhibitory activity of GIPL was also observed when DC were stimulated by CD40L, an endogenous stimulus that mimics the interaction between T cells and APC. Since proinflammatory cytokines are important to a proper innate immunity and costimulatory molecules are directly involved in the induction of acquired immune response, our results suggest that T. cruzi GIPL may help parasite survival in the human host. It has been shown that T. cruzi infection inhibits basal production of IL-12 and TNF-α by human DC as well as profoundly impairs LPS-induced DC maturation (33). Interestingly, supernatants derived from suspensions of T. cruzi or T. cruzi-conditioned medium have similar inhibitory effects on LPS-induced cytokine release and costimulatory molecule surface expression, an effect attributed to trypanosomal immunosuppressive factor (19). In the present report, we have shown that GIPL from T. cruzi is able to induce the activities observed with the whole parasite.
The inhibitory activity induced by GIPL was not mediated through the induction of cell death. Cells remained viable, when analyzed by fluorescence-activated cell sorting, several days after culture. IL-8 secretion did not diminish in the presence of GIPL, reinforcing the argument that suppressive activity was not due to a total shutdown of cell functions.
T. cruzi GIPL has been shown to exhibit either stimulatory or inhibitory effects on different cell types from the host immune system, including macrophages (13) and NK cells (8) as well as T cells (3, 16) and B cells (4). While the ceramide portion of T. cruzi GIPL is responsible for inducing apoptosis on macrophages (13) and suppressing T cells (16), the oligosaccharide moiety from the same glycolipid leads to B-cell activation and Ig secretion in a nonspecific manner (4). It has also been shown that GIPL stimulates the specific humoral response characterized by high levels of specific IgM and IgG antibodies present in sera of patients with acute (32) and chronic (15) Chagas' disease, respectively. Considering that GIPL has no protein chain covalently linked to it, one might propose the involvement of nonconventional T cells that recognize glycolipids to promote Ig isotype switching from IgM to IgG (25). Different reports demonstrated that NK T cells recognize bacterial and synthetic glycolipids presented by human (12) and mouse (18) CD1 molecules. In fact, recent studies showed the ability of CD1 molecules to bind GPI anchor and GIPL from Plasmodium falciparum and Trypanosoma brucei (28). Therefore, it is possible that NK T cells have a primary role in promoting a major histocompatibility complex-unrestricted antibody response to GIPL and GPI anchor molecules during early stages of infection with different parasitic protozoa.
The ceramide portion of T. cruzi GIPL had marked effects on endocytosis, leading to intense vacuole formation in J774.G8 cells and murine peritoneal macrophages (30). In fact, similar vesicle formation, mostly containing GIPL, is observed in macrophages treated with GIPL as early as 1 h after addition to the cultures (F. Ribeiro-Gomes, personal communication). A proteophosphoglycan secreted by Leishmania mexicana also induces vacuole formation in macrophages (21), suggesting a common set of biologic activities exerted by this related family of parasite molecules. It is possible that the inhibitory results observed in our study are related to this kind of effect, which could contribute to precluding the secretion of cytokines rather than interfering in transduction signals responsible for cytokine production. On the other hand, it has been shown that GPI anchors and GIPLs from T. cruzi are able to bind to Toll-like receptor 2 (TLR-2) on CHO-K1 cells transfected with CD14 and TLR-2. Macrophages from TLR-2 knockout or TLR-4 knockout mice showed that TLR-2 expression appears to be essential for induction of IL-12, TNF-α, and NO by GPI anchors (6). Better characterization of such pathways may help to determine key points that could be used by the parasites to escape immune surveillance.
The suppressive activity of GIPL on human APC does not seem to alter the lymphocytic response from patients with Chagas' disease (data not shown). This fact points out the importance of this molecule in the beginning stage, but not later stages, of infection by T. cruzi and the mechanisms used by the parasite to subvert the innate response and establish infection. Early interactions between protozoa and host innate immune system are of crucial importance in determining the fate of parasite-host relationship. The complex composition of GIPL exhibiting both inhibitory and stimulatory moieties makes it difficult to foresee what might be the predominant effect on a natural infection. It may depend on parasite numbers or other yet-unknown factors. It is certain, however, that the suppressive activities of GIPL predominate on human APC and that the shown immunoregulatory activities of protozoan-derived GIPL are potentially important in determining evasion of the host immune response. The role of these molecules in the pathogenesis of infection with protozoan parasites deserves further scrutiny.
Acknowledgments
We thank Silvia Cardoso and Jorge Clarêncio for technical assistance.
This work was supported by NIH grant AI 30639, PAPES/FIOCRUZ, and Pronex/CNPq, Instituto de Investigaçion en Immunologia.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aliberti, J. C., M. A. Cardoso, G. A. Martins, R. T. Gazzinelli, L. Q. Vieira, and J. S. Silva. 1996. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect. Immun. 64:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Bellio, M., A. C. Liveira, C. S. Mermelstein, M. A. Capella, J. P. Viola, J. P. Levraud, G. A. Dosreis, J. O. Previato, and L. Mendonca-Previato. 1999. Costimulatory action of glycoinositolphospholipids from Trypanosoma cruzi: increased interleukin 2 secretion and induction of nuclear translocation of the nuclear factor of activated T cells 1. FASEB J. 13:1627-1636. [DOI] [PubMed] [Google Scholar]
- 4.Bento, C. A., M. B. Melo, J. O. Previato, L. Mendonca-Previato, and L. M. Pecanha. 1996. Glycoinositolphospholipids purified from Trypanosoma cruzi stimulate Ig production in vitro. J. Immunol. 157:4996-5001. [PubMed] [Google Scholar]
- 5.Camargo, M. M., I. C. Almeida, M. E. Pereira, M. A. Ferguson, L. R. Travassos, and R. T. Gazzinelli. 1997. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J. Immunol. 158:5890-5901. [PubMed] [Google Scholar]
- 6.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 7.Carreira, J. C., C. Jones, R. Wait, J. O. Previato, and L. Mendonca-Previato. 1996. Structural variation in the glycoinositolphospholipids of different strains of Trypanosoma cruzi. Glycoconj. J. 13:955-966. [DOI] [PubMed] [Google Scholar]
- 8.De Arruda Hinds, L. B., L. M. Previato, J. O. Previato, Q. Vos, J. J. Mond, and L. M. Pecanha. 1999. Modulation of B-lymphocyte and NK cell activities by glycoinositolphospholipid purified from Trypanosoma cruzi. Infect. Immun. 67:6177-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lederkremer, R. M., C. Lima, M. I. Ramirez, M. A. Ferguson, S. W. Homans, and J. Thomas-Oates. 1991. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi epimastigotes. J. Biol. Chem. 266:23670-23675. [PubMed] [Google Scholar]
- 10.de Lederkremer, R. M., C. E. Lima, M. I. Ramirez, M. F. Goncalvez, and W. Colli. 1993. Hexadecylpalmitoylglycerol or ceramide is linked to similar glycophosphoinositol anchor-like structures in Trypanosoma cruzi. Eur. J. Biochem. 218:929-936. [DOI] [PubMed] [Google Scholar]
- 11.Descoteaux, A., S. J. Turco, D. L. Sacks, and G. Matlashewski. 1991. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J. Immunol. 146:2747-2753. [PubMed] [Google Scholar]
- 12.Ernst, W. A., J. Maher, S. Cho, K. R. Niazi, D. Chatterjee, D. B. Moody, G. S. Besra, Y. Watanabe, P. E. Jensen, S. A. Porcelli, M. Kronenberg, and R. L. Modlin. 1998. Molecular interaction of CD1b with lipoglycan antigens. Immunity 8:331-340. [DOI] [PubMed] [Google Scholar]
- 13.Freire-de-Lima, C. G., M. P. Nunes, S. Corte-Real, M. P. Soares, J. O. Previato, L. Mendonca-Previato, and G. A. DosReis. 1998. Proapoptotic activity of a Trypanosoma cruzi ceramide-containing glycolipid turned on in host macrophages by IFN-gamma. J. Immunol. 161:4909-4916. [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., M. M. Camargo, I. C. Almeida, Y. S. Morita, M. Giraldo, A. Acosta-Serrano, S. Hieny, P. T. Englund, M. A. Ferguson, L. R. Travassos, and A. Sher. 1997. Identification and characterization of protozoan products that trigger the synthesis of IL-12 by inflammatory macrophages. Chem. Immunol. 68:136-152. [DOI] [PubMed] [Google Scholar]
- 15.Golgher, D. B., W. Colli, T. Souto-Padron, and B. Zingales. 1993. Galactofuranose-containing glycoconjugates of epimastigote and trypomastigote forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 60:249-264. [DOI] [PubMed] [Google Scholar]
- 16.Gomes, N. A., J. O. Previato, B. Zingales, L. Mendonca-Previato, and G. A. DosReis. 1996. Down-regulation of T lymphocyte activation in vitro and in vivo induced by glycoinositolphospholipids from Trypanosoma cruzi. Assignment of the T cell-suppressive determinant to the ceramide domain. J. Immunol. 156:628-635. [PubMed] [Google Scholar]
- 17.Hunter, C. A., T. Slifer, and F. Araujo. 1996. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect. Immun. 64:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joyce, S., A. S. Woods, J. W. Yewdell, J. R. Bennink, A. D. De Silva, A. Boesteanu, S. P. Balk, R. J. Cotter, and R. R. Brutkiewicz. 1998. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 279:1541-1544. [DOI] [PubMed] [Google Scholar]
- 19.Kierszenbaum, F., S. Majumder, P. Paredes, M. K. Tanner, and M. B. Sztein. 1998. The Trypanosoma cruzi immunosuppressive factor (TIF) targets a lymphocyte activation event subsequent to increased intracellular calcium ion concentration and translocation of protein kinase C but previous to cyclin D2 and cdk4 mRNA accumulation. Mol. Biochem. Parasitol. 92:133-145. [DOI] [PubMed] [Google Scholar]
- 20.McNeely, T. B., and S. J. Turco. 1990. Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J. Immunol. 144:2745-2750. [PubMed] [Google Scholar]
- 21.Peters, C., Y. D. Stierhof, and T. Ilg. 1997. Proteophosphoglycan secreted by Leishmania mexicana amastigotes causes vacuole formation in macrophages. Infect. Immun. 65:783-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Previato, J. O., P. A. Gorin, M. Mazurek, M. T. Xavier, B. Fournet, J. M. Wieruszesk, and L. Mendonca-Previato. 1990. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J. Biol. Chem. 265:2518-2526. [PubMed] [Google Scholar]
- 23.Previato, J. O., R. Wait, C. Jones, and L. Mendonca-Previato. 1994. Structural analysis of novel rhamnose-branched oligosaccharides from the glycophosphosphingolipids of Leptomonas samueli. Glycoconj. J. 11:23-33. [DOI] [PubMed] [Google Scholar]
- 24.Romani, L., P. Puccetti, and F. Bistoni. 1997. Interleukin-12 in infectious diseases. Clin. Microbiol. Rev. 10:611-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ropert, C., and R. T. Gazzinelli. 2000. Signaling of immune system cells by glycosylphosphatidylinositol (GPI) anchor and related structures derived from parasitic protozoa. Curr. Opin. Microbiol. 3:395-403. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenkman, S., M. A. Ferguson, N. Heise, M. L. de Almeida, R. A. Mortara, and N. Yoshida. 1993. Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 59:293-303. [DOI] [PubMed] [Google Scholar]
- 28.Schofield, L., M. J. McConville, D. Hansen, A. S. Campbell, B. Fraser-Reid, M. J. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 29.Serrano, A. A., S. Schenkman, N. Yoshida, A. Mehlert, J. M. Richardson, and M. A. Ferguson. 1995. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J. Biol. Chem. 270:27244-27253. [DOI] [PubMed] [Google Scholar]
- 30.Smith, S. W., and R. L. Lester. 1974. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J. Biol. Chem. 249:3395-3405. [PubMed] [Google Scholar]
- 31.Tarleton, R. L. 1988. Tumour necrosis factor (cachectin) production during experimental Chagas' disease. Clin. Exp. Immunol. 73:186-190. [PMC free article] [PubMed] [Google Scholar]
- 32.Umezawa, E. S., M. A. Shikanai-Yasuda, A. Gruber, V. L. Pereira-Chioccola, and B. Zingales. 1996. Trypanosoma cruzi defined antigens in the serological evaluation of an outbreak of acute Chagas disease in Brazil (Catole do Rocha, Paraiba). Mem. Inst. Oswaldo Cruz 91:87-93. [DOI] [PubMed] [Google Scholar]
- 33.Van Overtvelt, L., N. Vanderheyde, V. Verhasselt, J. Ismaili, L. De Vos, M. Goldman, F. Willems, and B. Vray. 1999. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect. Immun. 67:4033-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhasselt, V., C. Buelens, F. Willems, D. De Groote, N. Haeffner-Cavaillon, and M. Goldman. 1997. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 158:2919-2925. [PubMed] [Google Scholar]