Abstract
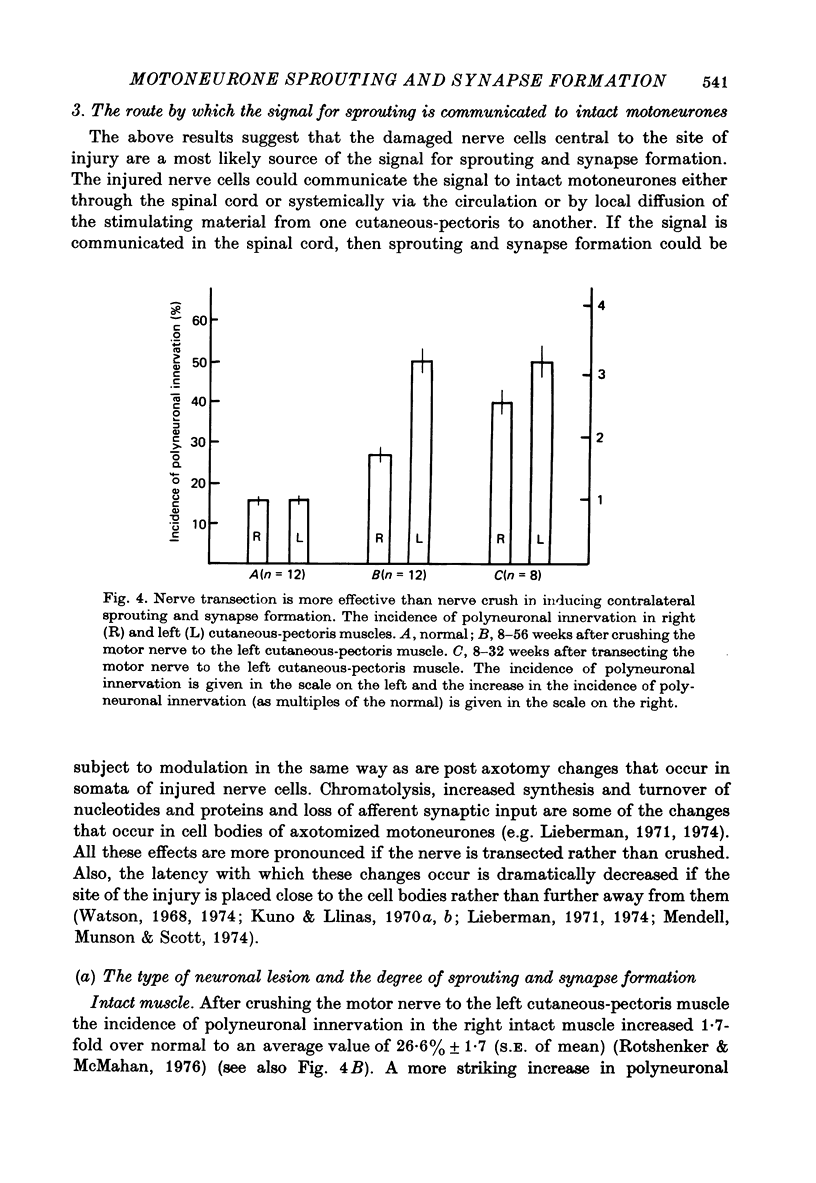
1. Denervation of one cutaneous-pectoris muscle of the frog induces the formation of new synapses in the intact innervated muscle on the opposite side. After crushing the motor nerve to the left muscle the incidence of polyneuronal innervation in the right intact muscle increased from an average normal value of 16% to an average value of 27% (Rotshenker & McMahan, 1976).
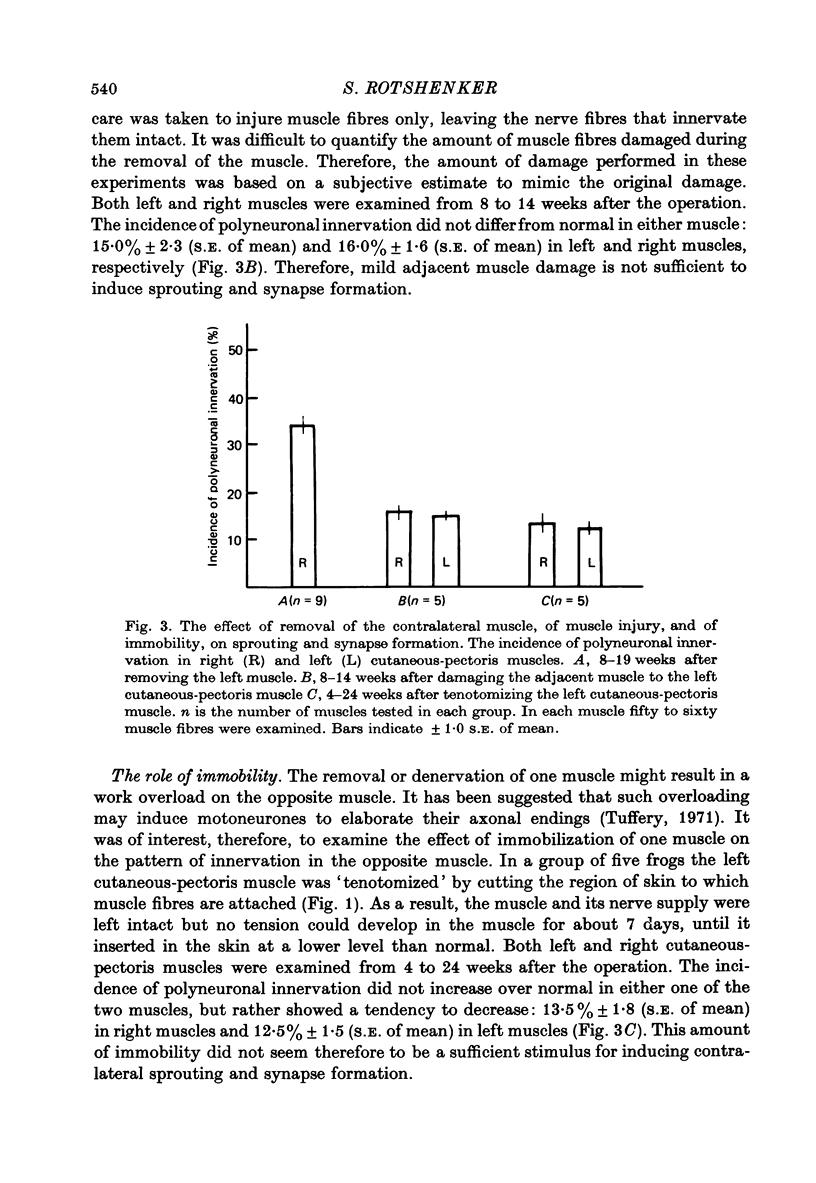
2. The formation of the new synapses in the intact muscle is independent of the presence of denervated muscle fibres or degenerating axons peripheral to the site of axotomy. After removing the left cutaneous-pectoris muscle, the proportion of polyneuronally innervated muscle fibres in the right intact muscle increased to an average value of 34%.
3. The number of new synapses formed in one muscle is dependent upon the type of the lesion to the motor nerve to the opposite muscle; 40% of the muscle fibres on the right side were found to be polyneuronally innervated after transecting the motor nerve on the opposite side, as compared to 27% after crushing it.
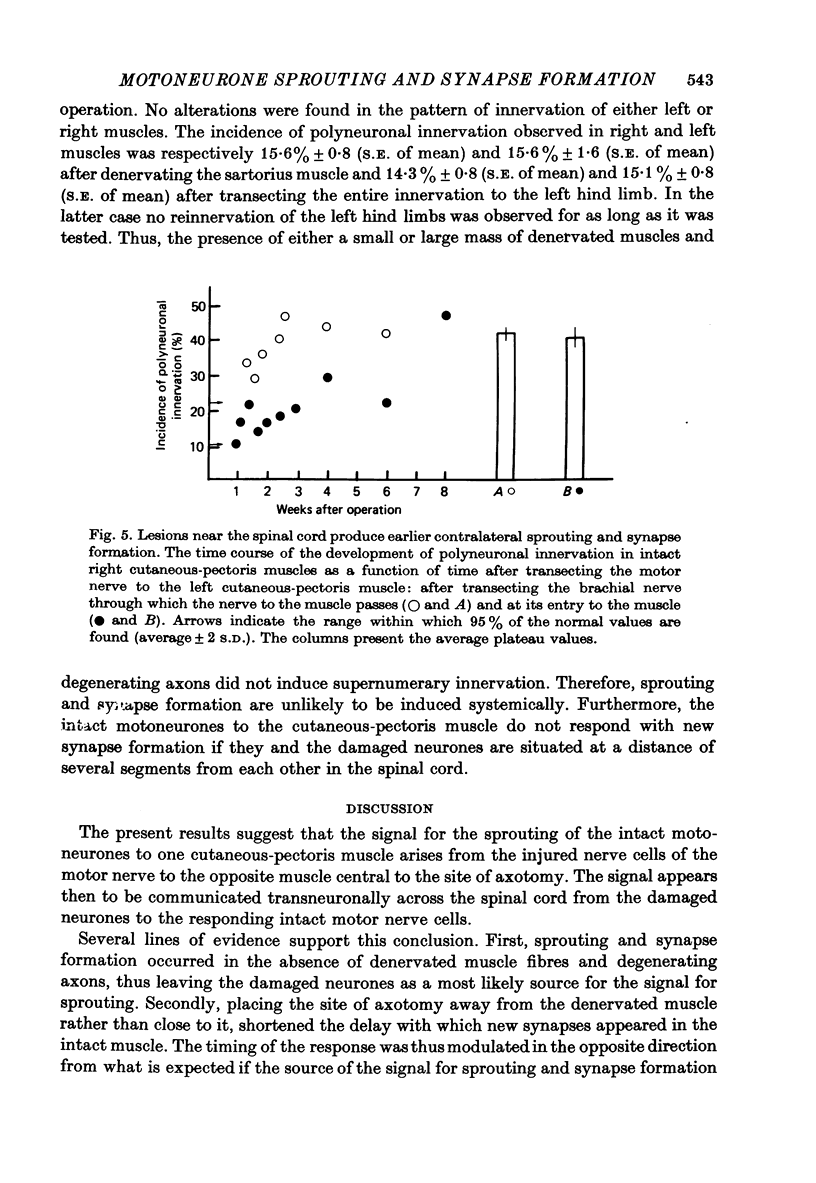
4. The delay with which new synapses are formed on the unoperated side is dependent upon the distance from the spinal cord of the axotomy. New synapses were detected 4-8 weeks after cutting the opposite nerve close to the muscle. Placing the site of axotomy close to the spinal cord shortened the delay and new synapses were detected as early as 9 days after the operation.
5. The stimulus for the formation of new synapses by an intact nerve is ineffective if the injured nerve on the contralateral side originates from distant segments of the spinal cord. The pattern of innervation in cutaneous-pectoris muscles was not altered following denervation of distant muscles in the hind limb.
6. These results suggest that the signal for sprouting and synapse formation may arise in the damaged nerve cells, central to the site of axotomy, and then be communicated transneuronally within the spinal cord to the intact motoneurones on the opposite side.
Full text
PDF


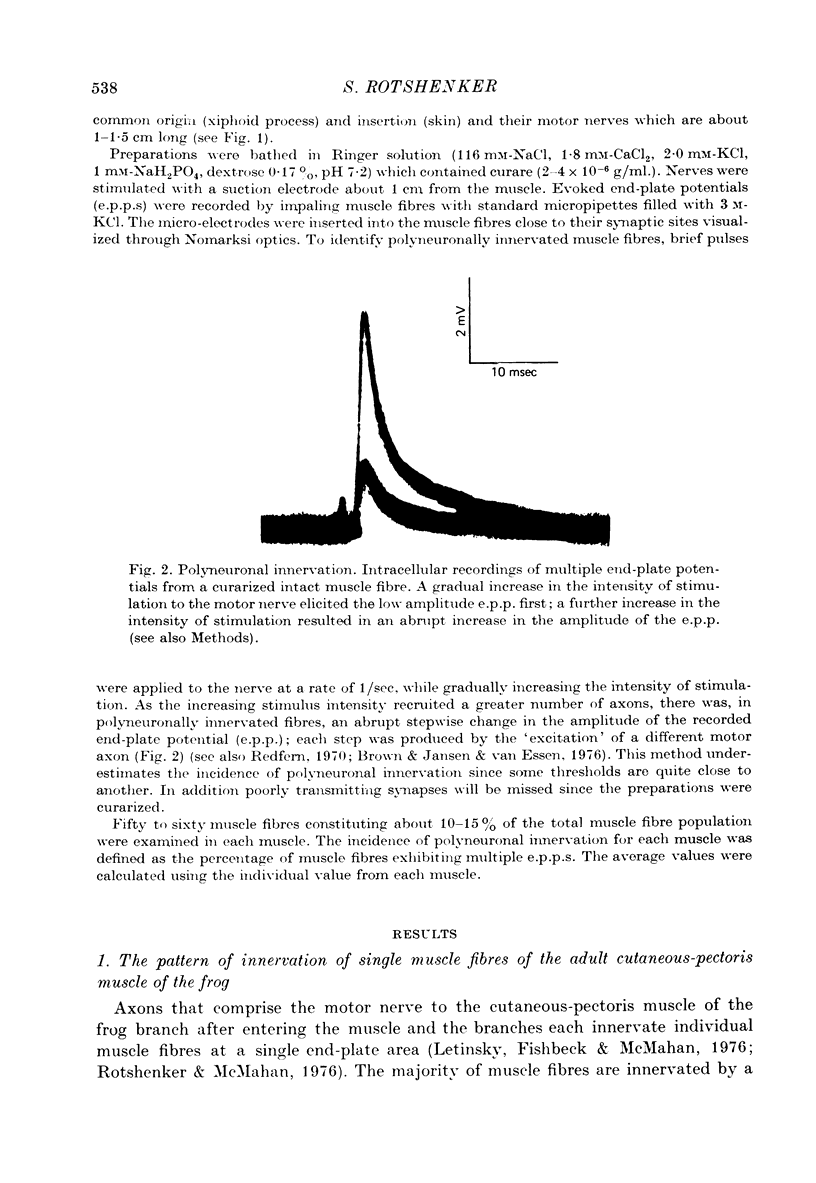






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar C. E., Bisby M. A., Cooper E., Diamond J. Evidence that axoplasmic transport of trophic factors is involved in the regulation of peripheral nerve fields in salamanders. J Physiol. 1973 Oct;234(2):449–464. doi: 10.1113/jphysiol.1973.sp010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of blocking the nerve with a local anaesthetic on the evolution of multiinnervation at the regenerating neuromuscular junction of the rat. Brain Res. 1978 Jun 23;149(1):89–96. doi: 10.1016/0006-8993(78)90589-9. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977 Feb 3;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull R. E. Rôle of axonal transport in maintaining central synaptic connections. Exp Brain Res. 1975 Nov 28;24(1):97–101. doi: 10.1007/BF00236020. [DOI] [PubMed] [Google Scholar]
- Cull R. E. Rôle of nerve-muscle contact in maintaining synaptic connections. Exp Brain Res. 1974;20(3):307–310. doi: 10.1007/BF00238321. [DOI] [PubMed] [Google Scholar]
- Davey B., Younkin S. G. Effect of nerve stump length on cholinesterase in denervated rat diaphragm. Exp Neurol. 1978 Mar;59(1):168–175. doi: 10.1016/0014-4886(78)90210-8. [DOI] [PubMed] [Google Scholar]
- Diamond J., Cooper E., Turner C., Macintyre L. Trophic regulation of nerve sprouting. Science. 1976 Jul 30;193(4251):371–377. doi: 10.1126/science.935873. [DOI] [PubMed] [Google Scholar]
- EDDS M. V., Jr Collateral nerve regeneration. Q Rev Biol. 1953 Sep;28(3):260–276. doi: 10.1086/399699. [DOI] [PubMed] [Google Scholar]
- GUTH L. Neuromuscular function after regeneration of interrupted nerve fibers into partially denervated muscle. Exp Neurol. 1962 Aug;6:129–141. doi: 10.1016/0014-4886(62)90083-3. [DOI] [PubMed] [Google Scholar]
- Guth L. Axonal regeneration and functional plasticity in the central nervous system. Exp Neurol. 1974 Dec;45(3):606–654. doi: 10.1016/0014-4886(74)90165-4. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. A study of the factors influencing innervation of muscles by implanted nerves. Aust J Exp Biol Med Sci. 1951 Jul;29(4):289–308. doi: 10.1038/icb.1951.35. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. Local re-innervation in partially denervated muscle; a histophysiological study. Aust J Exp Biol Med Sci. 1950 Jul;28(4):383–397. doi: 10.1038/icb.1950.39. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H., SPRINGELL P. H. An attempt at the chemical identification of neurocletin (the substance evoking axon-sprouting. Aust J Exp Biol Med Sci. 1951 Nov;29(6):417–424. doi: 10.1038/icb.1951.47. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Nerve stump length and membrane changes in denervated skeletal muscle. Nat New Biol. 1972 Mar 15;236(63):60–61. doi: 10.1038/newbio236060a0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCO J. V., EYZAGUIRRE C. Fibrillation and hypersensitivity to ACh in denervated muscle: effect of length of degenerating nerve fibers. J Neurophysiol. 1955 Jan;18(1):65–73. doi: 10.1152/jn.1955.18.1.65. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S., Fischbeck K. H., McMahan U. J. Precision of reinnervation of original postsynaptic sites in frog muscle after a nerve crush. J Neurocytol. 1976 Dec;5(6):691–718. doi: 10.1007/BF01181582. [DOI] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Munson J. B., Scott J. G. Connectivity changes of Ia afferents on axotomized motoneurons. Brain Res. 1974 Jun 20;73(2):338–342. doi: 10.1016/0006-8993(74)91054-3. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978 Apr;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following colchicine application to post-ganglionic nerves. J Physiol. 1976 Jul;259(1):159–175. doi: 10.1113/jphysiol.1976.sp011459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., McMahan U. J. Altered patterns of innervation in frog muscle after denervation. J Neurocytol. 1976 Dec;5(6):719–730. doi: 10.1007/BF01181583. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Sprouting of intact motor neurons induced by neuronal lesion in the absence of denervated muscle fibers and degenerating axons. Brain Res. 1978 Oct 27;155(2):354–356. doi: 10.1016/0006-8993(78)91029-6. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Sutherland F. I. Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol. 1973 Sep;2(3):315–328. doi: 10.1007/BF01104033. [DOI] [PubMed] [Google Scholar]
- Székely G. The morphology of motoneurons and dorsal root fibers in the frog's spinal cord. Brain Res. 1976 Feb 20;103(2):275–290. doi: 10.1016/0006-8993(76)90799-x. [DOI] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Cellular responses to axotomy and to related procedures. Br Med Bull. 1974 May;30(2):112–115. doi: 10.1093/oxfordjournals.bmb.a071179. [DOI] [PubMed] [Google Scholar]
- Watson W. E. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J Physiol. 1968 Jun;196(3):655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]


