Abstract
Resistance to reactive oxygen intermediates and reactive nitrogen intermediates in vitro of a clinical isolate of Mycobacterium tuberculosis (CDC1551) that caused a large outbreak of tuberculosis was compared to that of M. tuberculosis strains CB3.3, H37Rv, H37Ra, Erdman, RJ2E, C.C. 13, and C.C. 22 as well as M. bovis strains Ravenel and BCG. CDC1551 and CB3.3 were significantly more resistant to both hydrogen peroxide (H2O2) and acidified sodium nitrite than were the other strains tested. This biological phenotype may serve as an in vitro marker for clinical strains of M. tuberculosis likely to cause a large outbreak of tuberculosis.
A strain of Mycobacterium tuberculosis (CDC1551 or CSU 93) was responsible for a recent outbreak of tuberculosis (TB) in a rural area near the Kentucky-Tennessee border (29). In a population at low risk for TB, 21 persons were diagnosed with active TB between 1994 and 1996. Among 429 contacts of TB index patients, 72% had positive tuberculin reactions, including 20% with documented skin test conversions (29). Because of the high number of positive tuberculin reactions, skin test conversions, and infected casual contacts, it was suggested that CDC1551 is an exceptionally virulent strain of M. tuberculosis (29).
The role of reactive oxygen intermediates (ROI) and, more recently, reactive nitrogen intermediates (RNI) in host defense against M. tuberculosis has been studied in various murine models (2, 3, 5-7, 10, 11, 13, 19, 28). Although the importance of RNI defense against M. tuberculosis has been well established in the murine model, accumulating evidence suggests that RNI may play a role in host defense against M. tuberculosis in humans (16, 22, 30). Numerous in vitro studies demonstrate that strains of M. tuberculosis vary with regard to their susceptibility to ROI (15, 18, 20) and RNI (12, 24, 26, 31). In addition, several M. tuberculosis genes associated with RNI resistance have been recently described (4, 8, 27). One example of an RNI-resistant M. tuberculosis strain is the drug-susceptible, clinical strain CB3.3 (C strain) that was involved in a New York City outbreak between 1991 and 1994. Investigations of CB3.3 found that it was more resistant to acidified sodium nitrite (ASN), a chemical reaction that converts nitrite to reactive nitrogen species, than were H37Rv and several other clinical M. tuberculosis isolates (12). Infection with strain CB3.3 was epidemiologically associated with injection drug users with tuberculosis in New York City (12).
In order to determine if the clinical strain CDC1551 exhibited characteristics similar to those of CB3.3, we compared its RNI and ROI resistance patterns to those of other clinical isolates, including CB3.3 (from New York City), RJ2E (one of the predominant M. tuberculosis strains circulating in Rio de Janeiro, Brazil) (9), and C.C. 13 and C.C. 22 (from northern California) as well as virulent (H37Rv, Erdman, and M. bovis Ravenel) and attenuated (H37Ra and M. bovis BCG) reference laboratory M. tuberculosis complex strains.
All strains of mycobacteria were grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, Mich.) with 0.5% glycerol, 0.02% Tween 80, and ADC enrichment (Difco Laboratories) to mid-log phase. Multiple cultures from the original slant of each strain were grown to similar absorbances, and then single cell suspensions of each isolate were prepared according to a previously published method (14). Multiple aliquots of each strain's single cell suspension were stored at −80°C for no longer than 3 months before use. The approximate number of organisms per frozen suspension was quantified by enumeration of CFU of serial dilutions plated on Middlebrook 7H11 agar. Survival was determined by placing 0.1 ml of thawed suspension into 0.9 ml of 7H9 broth (pH 5.4 for ASN assays or pH 7.0 for H2O2 assays) containing various concentrations of either sodium nitrite (Sigma, St. Louis, Mo.) or H2O2 (Sigma). As a control, a 0.1-ml aliquot from the same thawed suspension was added to 0.9 ml of 7H9 broth at the appropriate pH without sodium nitrite or H2O2. Each suspension was incubated at 37°C for 16 h, plated onto Middlebrook 7H11 agar (Difco Laboratories), and incubated at 37°C for 3 weeks. Resistance to the reagents was expressed as a proportion of the number of CFU recovered from isolates exposed to either ASN or H2O2 to the number of CFU recovered from the same isolates not exposed to these reagents. Each strain was tested similarly in triplicate. The data represent results from triplicate experiments from a single cell suspension per culture from at least three separate cultures for each strain. Statistical analysis was performed with the Student t test with the P value adjusted to <0.0055 by using Bonferroni's correction for multiple comparisons.
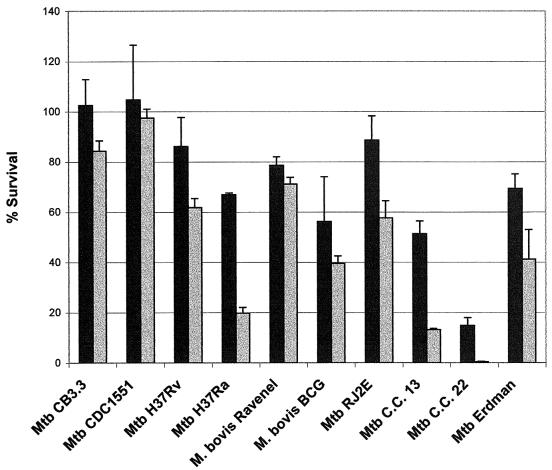
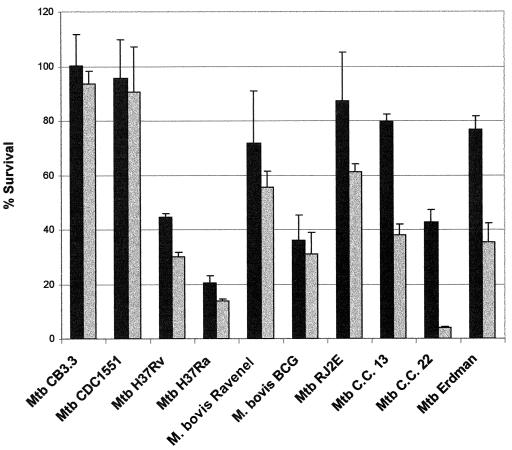
Aside from M. tuberculosis H37Ra and C.C. 22, most strains were relatively resistant to low concentrations of either H2O2 (2 mM) or ASN (3 mM) (Fig. 1 and 2). At 5 mM H2O2, CDC1551 was significantly (P < 0.0055) more resistant than were H37Rv, H37Ra, BCG, C.C. 13, and C.C. 22 (Table 1). Although not statistically significant, CDC1551 demonstrated greater resistance than did CB3.3, RJ2E, Erdman, and Ravenel (Fig. 1). Survival at 6 mM ASN indicated that CDC1551 was significantly (P < 0.0055) more resistant than were H37Ra, BCG, and C.C. 22 (Table 1). In addition, CDC1551 demonstrated enhanced resistance in 6 mM ASN compared to that of H37Rv, C.C. 13, and Erdman, although the levels were not statistically significant (P = 0.0068, 0.0059, and 0.006, respectively) (Table 1). Moreover, the percent survival of both CB3.3 and CDC1551 in 5 mM H2O2 and 6 mM ASN was higher than that of the eight other strains tested in this study (Fig. 1 and 2).
FIG. 1.
Effect of H2O2 on mycobacterial viability in Middlebrook 7H9 medium at 2 mM (black bars) and 5 mM (gray bars) concentrations. Data shown are representative of triplicate experiments. Results are expressed as percents survival relative to baseline ± standard errors of the means and are mean values of triplicate cultures for each strain.
FIG. 2.
Effect of ASN on mycobacterial viability in Middlebrook 7H9 medium at 3 mM (black bars) and 6 mM (gray bars) concentrations. Data shown are representative of triplicate experiments. Results are expressed as percents survival relative to baseline ± standard errors of the means and are mean values of triplicate cultures for each strain.
TABLE 1.
Comparison (P value) of resistance to ASN and H2O2 between CDC1551 and other mycobacterial strainsa
| Strain |
P value with reagent at concn
|
|||
|---|---|---|---|---|
| ASN
|
H2O2
|
|||
| 3 mM | 6 mM | 2 mM | 5 mM | |
| CB3.3 | ∗ | ∗ | ∗ | ∗ |
| H37Rv | ∗ | ∗ | ∗ | 0.0016 |
| H37Ra | ∗ | 0.0013 | ∗ | <0.001 |
| Ravenel | ∗ | ∗ | ∗ | ∗ |
| BCG | ∗ | 0.0049 | ∗ | 0.003 |
| RJ2E | ∗ | ∗ | ∗ | ∗ |
| C.C. 13 | ∗ | ∗ | ∗ | <0.001 |
| C.C. 22 | ∗ | <0.001 | ∗ | <0.001 |
| Erdman | ∗ | ∗ | ∗ | ∗ |
P values were determined by using Student's t test (significance levels were adjusted with Bonferroni's correction for multiple comparisons). ∗, not significant (P > 0.0055).
It was initially reported that mice infected with CDC1551 contained 10-fold-more bacilli per lung after 10 days and 100-fold-more bacilli per lung after 20 days than did mice infected with M. tuberculosis strain Erdman (29). Another study, however, observed that CDC1551 had similar growth rates in mouse lungs over 20 days of infection as H37Rv and M. bovis Ravenel and, therefore, concluded that CDC1551 is not necessarily a more virulent strain in the mouse model (23). More recent studies have demonstrated that CDC1551 grows similarly in vitro (18, 20, 21) and in vivo (1, 17, 21, 23) as H37Rv. In addition, a study by Orme demonstrated that CDC1551 grows similarly in mice as other clinical M. tuberculosis isolates associated with outbreaks (25). However, it has been demonstrated elsewhere that CDC1551 induces granulomas in mouse lungs earlier than does H37Rv, Erdman, or two clinical isolates and that higher levels of tumor necrosis factor alpha, interleukin 10 (IL-10), IL-6, and IL-12 were induced in monocytes after infection with CDC1551 or by exposure of monocytes to lipid fractions from CDC1551 (21). Consequently, it was suggested that CDC1551 was not more virulent than other M. tuberculosis isolates in terms of growth in mice but might be more immunogenic (21).
RNI are an important determinant of infection outcome in the interaction between M. tuberculosis and the mouse macrophage. Mice disrupted in the inducible nitric oxide synthase (NOS2) gene have increased susceptibility to M. tuberculosis infection (19). More recently, Thoma-Uszynski et al. demonstrated that the activation of toll-like receptor 2 (TLR2) leads to killing of intracellular M. tuberculosis in both human and murine macrophages (28). They suggested that killing in murine macrophages is mediated by the nitric oxide-dependent pathway but that in human macrophages it is independent of this pathway (28). Consequently, the role of RNI in controlling M. tuberculosis infection in the human host continues to be debated, although several studies have provided evidence that RNI are expressed by M. tuberculosis-infected human alveolar macrophages (16, 22, 30).
The possible epidemiological importance of RNI resistance in M. tuberculosis was first observed with the demonstration that the most common drug-susceptible strain of M. tuberculosis circulating in New York City during the early 1990s was highly resistant to RNI (12). A recent study compared CDC1551 to seven clinical isolates of M. tuberculosis for susceptibility to chemically generated RNI in vitro and was unable to demonstrate any significant differences regarding RNI susceptibility among these strains (32). It should be noted that a laboratory reference strain (e.g., H37Rv) or a clinical strain with known levels of RNI resistance was not included in that study for comparison.
Our results demonstrate that, at lower concentrations of ASN and H2O2 (3 and 2 mM, respectively), most strains of mycobacteria were relatively resistant, except for the attenuated, laboratory strain of M. tuberculosis (H37Ra) and a clinical isolate of M. tuberculosis (C.C. 22). These results are consistent with those of Rhoades and Orme (26), who demonstrated that certain strains could resist some degree of RNI generated in vitro. At higher concentrations of ASN (6 mM), however, the CDC1551 strain was as resistant as the previously described RNI-resistant clinical isolate CB3.3 from New York City (12). The percent survival of CB3.3 as well as H37Rv at 6 mM ASN was in the same range as that reported for these strains by Friedman et al. (12), thus supporting the reproducibility of the ASN assay. Moreover, CDC1551 was the most resistant strain among the 10 strains tested when exposed to 5 mM H2O2. Although our observation is preliminary, based on a small number of isolates, high-level resistance to RNI and ROI may serve as a convenient marker of M. tuberculosis strains with an epidemic potential. Opportunities to examine this suggestion may arise with large outbreaks of tuberculosis that may occur in the future.
Acknowledgments
This study was supported by NIH grant HL51967 to L.W.R. and by the John P. Dowdle Endowment grant GH32 and NIH fellowship DA05874 to M.A.F.
We thank Lisa Morici and Amee Manges for technical assistance and helpful discussions. We also thank Ed Desmond, Jennifer Flood, and Lisa Pascopella at the California State Health Department and Sally Cantrell for providing C.C. 13 and C.C. 22 and T. M. Shinnick, Division of Bacterial Diseases, Centers for Disease Control and Prevention, Atlanta, Ga., for kindly providing CDC1551.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bishai, W. R., A. M. Dannenberg, Jr., N. Parrish, R. Ruiz, P. Chen, B. C. Zook, W. Johnson, J. W. Boles, and M. L. M. Pitt. 1999. Virulence of Mycobacterium tuberculosis CDC1551 and H37Rv in rabbits evaluated by Lurie's pulmonary tubercle count method. Infect. Immun. 67:4931-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide synthase inhibitors in murine infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L., Q. W. Xie, and C. Nathan. 1998. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol. Cell 1:795-805. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 7.Denis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via generation of reactive nitrogen intermediate. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 8.Ehrt, S., M. U. Shiloh, J. Ruan, M. Choi, S. Gunzberg, C. Nathan, Q. W. Xie, and L. W. Riley. 1997. A novel antioxidant gene from Mycobacterium tuberculosis. J. Exp. Med. 186:1885-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fandinho, F. C. O., A. L. Kritski, C. Hofer, H. Conde, Jr., R. M. C. Ferreira, M. G. Saad, L. W. Riley, and L. S. Fonseca. 2000. RFLP patterns and risk factors for resistant tuberculosis transmission among hospitalized patients in Rio de Janeiro, Brazil. Trans. R. Soc. Trop. Med. Hyg. 94:271-275. [DOI] [PubMed] [Google Scholar]
- 10.Flesch, I. E. A., and S. H. E. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman, C. R., G. C. Quinn, B. N. Kreiswirth, D. C. Perlman, N. Salomon, N. Schulger, M. Lutfey, J. Berger, N. Poltoratskaia, and L. W. Riley. 1997. Widespread dissemination of a drug-susceptible strain of Mycobacterium tuberculosis. J. Infect. Dis. 176:478-484. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, I., R. Guler, D. Vesin, M. L. Olleros, P. Vassalli, Y. Chvatchko, M. Jacobs, and B. Ryffel. 2000. Lethal Mycobacterium bovis bacillus Calmette Guerin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab. Investig. 80:1385-1397. [DOI] [PubMed] [Google Scholar]
- 14.Grover, A. A., H. K. Kim, E. H. Weigeshaus, and D. W. Smith. 1967. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70°C. J. Bacteriol. 94:832-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackett, P. S., V. R. Aber, and D. B. Lowrie. 1978. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J. Gen. Microbiol. 104:37-45. [DOI] [PubMed] [Google Scholar]
- 16.Jagannath, C., J. K. Actor, and R. L. Hunter, Jr. 1998. Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide Biol. Chem. 2:174-186. [DOI] [PubMed] [Google Scholar]
- 17.Kelley, C. L., and F. M. Collins. 1999. Growth of a highly virulent strain of Mycobacterium tuberculosis in mice of differing susceptibility to tuberculous challenge. Tuber. Lung Dis. 79:367-370. [DOI] [PubMed] [Google Scholar]
- 18.Laochumroonvorapong, P., S. Paul, C. Manca, V. H. Freedman, and G. Kaplan. 1997. Mycobacterial growth and sensitivity to H2O2 killing in human monocytes in vitro. Infect. Immun. 65:4850-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMicking, J. D., R. J. North, R. LaCource, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manca, C., S. Paul, C. E. Barry III, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocytes in vitro. Infect. Immun. 67:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manca, C., L. Tsenova, C. E. Barry III, A. Bergtold, S. Freeman, P. A. J. Haslett, J. M. Musser, V. H. Freeman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but it is not more virulent than other clinical isolates. J. Immunol. 162:6740-6746. [PubMed] [Google Scholar]
- 22.Nicholson, S., M. G. Bonecini-Almeida, J. R. Lapa e Silva, C. Nathan, Q. W. Xie, R. Mumford, J. R. Weidner, J. Calaycay, J. Geng, N. Boechat, C. Linhares, W. Rom, and J. L. Ho. 1996. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J. Exp. Med. 183:2293-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North, R. J., L. Ryan, R. LaCource, T. Mogues, and M. E. Goodrich. 1999. Growth rate of mycobacteria in mice as an unreliable indicator of mycobacterial virulence. Infect. Immun. 67:5483-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien, L., J. Carmichael, D. B. Lowrie, and P. W. Andrews. 1994. Strains of Mycobacterium tuberculosis differ in susceptibility to reactive nitrogen intermediates in vitro. Infect. Immun. 62:5187-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orme, I. M. 1999. Virulence of recent notorious Mycobacterium tuberculosis isolates. Tuber. Lung Dis. 79:379-381. [DOI] [PubMed] [Google Scholar]
- 26.Rhoades, E. R., and I. M. Orme. 1997. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect. Immun. 65:1189-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan, J., G. St. John, S. Ehrt, L. Riley, and C. Nathan. 1999. noxR3, a novel gene from Mycobacterium tuberculosis, protects Salmonella typhimurium from nitrosative and oxidative stress. Infect. Immun. 67:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 29.Valway, S. E., M. P. C. Sanchez, T. F. Shinnick, I. Orme, T. Agerton, D. Hoy, J. S. Jones, H. Westmoreland, and I. M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633-639. [DOI] [PubMed] [Google Scholar]
- 30.Wang, C.-H., C.-Y. Liu, H.-C. Lin, C.-T. Yu, K. F. Chung, and H.-P. Kuo. 1998. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur. Respir. J. 11:809-815. [DOI] [PubMed] [Google Scholar]
- 31.Yu, K., C. Mitchell, Y. Xing, R. S. Magliozzo, B. R. Bloom, and J. Chan. 1999. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79:191-198. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, M., J. Gong, Z. Yang, B. Samten, M. D. Cave, and P. F. Barnes. 1999. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J. Infect. Dis. 179:1213-1217. [DOI] [PubMed] [Google Scholar]