Abstract
The importance of O-acetyl groups to the immunogenicity of Neisseria meningitidis serogroup A polysaccharide (PS) was examined in studies using human sera and mouse immunization. In 17 of 18 postimmunization human sera, inhibition enzyme-linked immunosorbent assay indicated that the majority of antibodies binding to serogroup A PS were specific for epitopes involving O-acetyl groups. Studies with mice also showed an essential role for O-acetyl groups, where serum bactericidal titers following immunization with de-O-acetylated (de-O-Ac) conjugate vaccine were at least 32-fold lower than those following immunization with O-Ac PS-conjugate vaccine and 4-fold lower than those following immunization with native capsular PS. Inhibition studies using native and de-O-Ac PS confirmed the specificity of murine antibodies to native PS. The dramatic reduction in immunogenicity associated with removal of O-acetyl groups indicates that O acetylation is essential to the immunogenic epitopes of serogroup A PS. Since levels of bactericidal antibodies are correlated with protection against disease, O-acetyl groups appear to be important in protection.
Neisseria meningitidis serogroup A causes epidemic meningococcal disease and is a major public health problem in many countries, particularly in the sub-Saharan “meningitis belt” region of Africa (1). In 1996 one of the largest recorded epidemics of serogroup A meningococcal meningitis occurred in the meningitis belt, with more than 180,000 reported cases (27). The occurrence of this and subsequent epidemics resulted in a renewed interest in vaccines for prevention of serogroup A meningococcal disease in Africa and elsewhere.
Capsular polysaccharide (PS) vaccines against serogroup A N. meningitidis have been licensed since 1976. Immunization with serogroup A PS elicits protective antibody responses. In some studies the PS has been shown to elicit an anamnestic response that is not typically seen with PS vaccines (9, 11). This vaccine has been effective in controlling serogroup A epidemics in Africa and other parts of the world (15, 23). However, the vaccination strategy using PS vaccine has several drawbacks. Previously, mass vaccination to prevent epidemic meningococcal disease was recommended when disease rates reached 15 cases per 100,000 for 2 weeks. When this strategy was used, fewer than half of the cases that would likely occur without intervention were prevented (27), and recently a lower epidemic threshold of 10 cases per 100,000 in 1 week has been recommended (16). Also, although protective levels of antibody have been shown to persist in adults for up to 10 years, the single dose of vaccine used in epidemic control provides protection of relatively short duration in young children (25, 32). For these reasons, efforts have been made to apply the concepts of PS-protein conjugation, which have been highly successful in controlling Haemophilus influenzae (4), to the development of meningococcal serogroup A vaccines.
Recently, serogroup C N. meningitidis conjugate vaccines were licensed in the United Kingdom. Three different serogroup C conjugate vaccines are now widely used there, resulting in a marked drop in the incidence of meningococcal disease in vaccinated individuals (24). Similar meningococcal serogroup A conjugate vaccines are under development. One of the earliest serogroup A conjugates to be studied was a bivalent meningococcal vaccine consisting of group A and C PSs covalently linked to CRM197 protein. This preparation, when studied in infants in The Gambia, failed to induce immunologic memory to the serogroup A component (13, 30). Lack of a memory response to the native PS could indicate that the primary vaccination with conjugate vaccine did not elicit a T-cell-dependent response. Alternatively, critical epitopes of the PS could have been altered during the conjugation process, such that the native PS used for boosting in toddlers was not recognized as antigenically similar to the protein-conjugated PS used for primary vaccinations during infancy.
The serogroup A N. meningitidis capsular PS is a homopolymer of N-acetyl-mannosamine-phosphate-linked α1-6. It is 70 to 95% O acetylated (O-Ac) at carbon 3 (10, 14). Capsular PSs from many pathogenic bacteria are O-Ac, and a common feature is that O-acetyl groups are easily removed at alkaline pH. Thus, O-acetyl groups could be partially removed in the process of PS activation and conjugation. Although the method used to prepare the conjugate vaccine used in the Gambian study did not appear to affect the O-acetyl groups at the stage of PS activation (5), the importance of O-acetyl groups in the immune response to vaccination with serogroup A N. meningitidis PS has not been fully characterized. An earlier study found reduced immunogenicity of de-O-Ac serogroup A PS in mice, but the results were confounded by the marked reduction in molecular size of the de-O-Ac PS (19). The present studies were undertaken to determine whether the presence of O-acetyl groups on meningococcal serogroup A PS is critical for the induction of a protective antibody response.
(This work was presented in part at the Uniformed Services Pediatric Society Meeting, 1998, and the 12th International Pathogenic Neisseria Conference, Galveston, Tex., November 2000.)
MATERIALS AND METHODS
Capsular and chemically modified PS.
Purified serogroup A N. meningitidis capsular PS was obtained from Aventis Pasteur-USA, Swiftwater, Pa. Molecular size was estimated using a Sepharose CL-4B gel filtration column eluted with a sodium acetate buffer (31). The degree of O acetylation was assessed by proton nuclear magnetic resonance (NMR) (14). Native PS was de-O acetylated by treatment with anhydrous hydrazine as previously described (12). Following precipitation and washing with acetone, the PS was dissolved in saline and then lyophilized. Comparison of the hydrazine-treated and native PSs by proton NMR confirmed complete removal of O-acetyl groups without other effects on the backbone PS structure. The molecular size of the PS was estimated by gel filtration as previously described. To evaluate the effect of PS size on binding specificity, moderately size-reduced O-Ac PS was prepared by mixing samples of PS with 0.01 N HCl at room temperature for 2 and 4 h. The reaction mixtures were neutralized, and the samples were dialyzed against water and lyophilized. The molecular size of the size-reduced PS was estimated by gel filtration.
Synthesis of PS conjugates with TT and HSA.
Meningococcal serogroup A PS or its de-O-Ac form (5 mg in 1 ml of H2O) was reacted with 2 mM NaIO4 at 4°C for 1.4 h. After quenching with 20 μl of 5 M glycerol and extensive dialysis against deionized water, the solution was mixed with 5 mg of tetanus toxoid (TT) or human serum albumin (HSA) and 0.2 ml of 10× phosphate-buffered saline (PBS) (pH 7.4) and lyophilized. The lyophilized sample was dissolved in 0.5 ml of H2O and reacted with 2 μl of pyridine borane for 4 days at room temperature. Ethanolamine at 3.3 M (pH 7) was added to the solution to a final concentration of 0.1 M and left for 3 h to block unreacted aldehyde groups. The conjugate was precipitated with 90% ammonium sulfate, which separated the conjugate from unconjugated PS. The precipitate was dissolved in 1× PBS (pH 7.4), dialyzed against 1× PBS (pH 7.4)-0.02% NaN3, and stored at 4°C. The contents of protein and PS in the conjugate were determined by the Bradford protein assay (Bio-Rad, Hercules, Calif.) and resorcinol assay (20), respectively.
Human antisera.
Pre- and postvaccination sera from nine adults previously immunized with N. meningitidis group A, C, Y, W-135 PS vaccine and nine children from Nepal who had received a serogroup A/C PS vaccine (2, 6) were examined by PS enzyme-linked immunosorbent assay (ELISA) and inhibition ELISA.
Mouse immunization.
This study was approved by and conducted according to guidelines of the Center for Biologics Evaluation and Research Animal Care and Use Committee. Groups of 10 female NIH/Swiss mice were immunized intraperitoneally at 4, 7, and 11 weeks of age with capsular PS vaccine (native PS) or de-O-Ac PS vaccine at 2 μg per dose or with PS conjugate vaccine (O-Ac-TT) or de-O-Ac PS conjugate vaccine (de-O-Ac-TT) at 1, 2, or 5 μg per dose; 5% Maalox was used as an adjuvant (28). Mice immunized with adjuvant alone served as controls. Serum samples were obtained prior to immunization, 3 weeks after the first immunization, and 4 weeks after the second and third immunizations. Pools of sera prepared by mixing equal volumes of individual sera were used in ELISAs with various coating antigens and in serum bactericidal assays (SBAs). The experiment was repeated (experiment 2) as described above except that only the 2-μg dose was used for the conjugate vaccines. Individual sera from this experiment were assayed by serogroup A PS ELISA, and pooled sera, prepared using equal volumes from each of 10 mice per group, were tested in SBA.
ELISA and inhibition ELISA.
The alkaline hydrolysis of the serogroup A meningococcal PS that removes the O-acetyl groups from the PS backbone also removes the lipid tails that are associated with PS. Previous studies have shown that the lipid tails are important for binding of serogroup C PS to ELISA plates with the methylated HSA (mHSA) method (2). Thus, to evaluate the specificity of binding of antibody to de-O-Ac PS, competitive inhibition assays were performed with native PS bound to the ELISA plates (PS ELISA) and de-O-Ac PS or native PS used to absorb specific antibodies.
For analysis of human sera, standard PS ELISA and inhibition ELISA were performed by the method of Arakere et al. (3) except for the use of Immulon no. 4 plates (Dynatek Technologies, Inc., Chantilly, Va.) and 0.01 M Tris-buffered saline-0.05% Tween 20 as the wash buffer. Fivefold dilutions of inhibiting PS starting from 50 μg/ml were incubated with a single serum dilution previously shown by ELISA to be in the linear range of the titration curve.
The PS ELISA and inhibition ELISA to determine mouse antibody levels were run essentially as they were for the human samples, using plates coated with native PS admixed with mHSA. In addition to being used in studies with native PS as the coating antigen, the murine antisera were also evaluated in assays using HSA conjugates of native and de-O-Ac PS as coating antigens to directly measure antibody binding to O-Ac and de-O-Ac PS. For all murine ELISAs, twofold dilutions of each individual serum or pooled sera starting at 1:50 in PBS-5% newborn calf serum-0.05% Tween 20 were assayed in duplicate. Goat anti-mouse antibodies, either specific for all isotypes of immunoglobulin (Ig) (alkaline phosphatase labeled; Kirkegaard & Perry, Gaithersburg, Md.) or isotype specific (horseradish peroxidase labeled; Southern Biotechnology Associates, Inc., Birmingham, Ala.), were diluted in PBS-5% newborn calf serum-0.05% Tween 20. The isotype-specific assays used ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Kirkegaard & Perry).
The antibody titer was calculated by comparison with a standard curve generated using a serum pool from animals immunized with O-Ac PS-TT conjugate that was arbitrarily assigned 1,000 ELISA units (EU). The coefficient of variation was consistently below 25%. For PS ELISA of individual mouse sera, the geometric mean titer in each experimental group was compared to that in the control group by Dunnett's test, using JMP statistical software (SAS Institute Inc., Cary, N.C.). For the isotype-specific assays, comparisons were based on the optical density (OD) reading. For inhibition studies, percent inhibition was calculated by comparison of the mean of two OD values for each inhibitor concentration to the mean of two OD values for sera and buffer only.
SBA.
The SBA was performed essentially as described previously (17) with minor modifications. The serogroup A strain F8238 was provided by George Carlone (Centers for Disease Control and Prevention, Atlanta, Ga.). The bacteria were diluted to approximately 80% transmittance at 530 nm and then further diluted to yield 50 to 80 CFU per 12.5 μl in Dulbecco's PBS with calcium, magnesium, and 0.1% gelatin. The pooled serum samples were tested in duplicate twice, using twofold serial dilutions as appropriate. Heat-inactivated complement was compared to active baby rabbit complement (Pel-Freeze Clinical Systems, LLC, Brown Deer, Wis.) to establish that the complement alone did not have endogenous bactericidal activity. CFU per well were enumerated under magnification, and the titer was defined as the highest dilution demonstrating at least a 50% reduction in the CFU compared to the numbers in wells containing complement, bacteria, and buffer only.
Disclaimer.
The opinions and assertions contained herein are those of the authors alone and do not reflect the views of the Uniformed Services University, the U.S. Army, the Department of Defense, or the Food and Drug Administration.
RESULTS
Characterization of de-O-Ac and size-reduced PS.
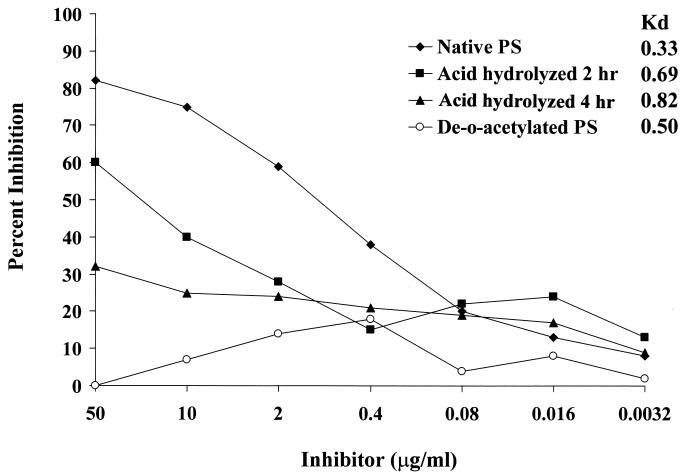
Elution profiles of the PS samples on Sepharose CL-4B yielded the following Kd values: native PS, 0.33; de-O-Ac PS, 0.50; and native PS hydrolyzed with HCl for 2 or 4 h, 0.69 and 0.82, respectively. Proton NMR spectra from PS samples before and after alkaline treatment confirmed that the O-acetyl groups were removed by the treatment and that the backbone PS structure remained unchanged (data not shown).
Binding specificity of human immune sera.
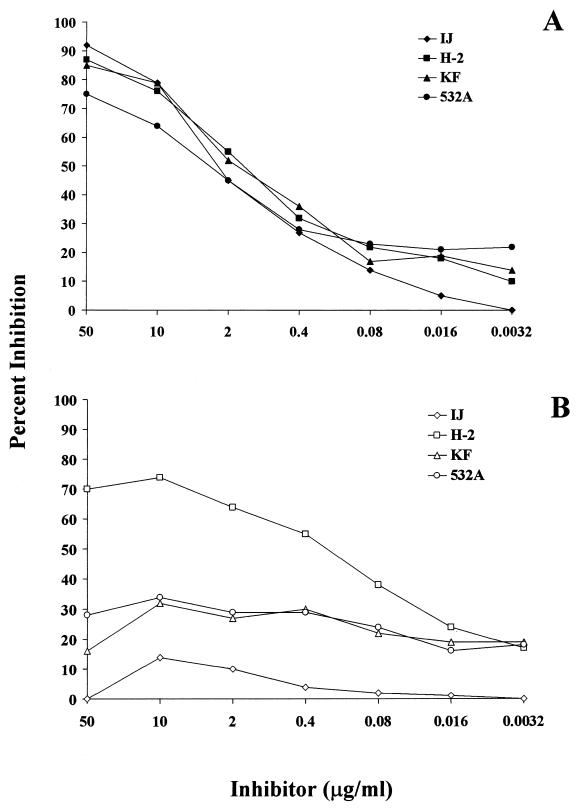
To evaluate the human responses to O-acetyl-associated epitopes on the serogroup A PS, binding of anti-serogroup A PS antibodies to either native or de-O-Ac PS was determined by using inhibition ELISA. Sera from 18 individuals who had responded to vaccination with a PS vaccine were tested. Incubation of all 18 serum samples with native PS at 50 μg/ml resulted in greater than 70% inhibition of antibody binding to the ELISA plates coated with native PS. Dilutions of the inhibiting PS yielded a dose-response curve; data from four representative sera are shown in Fig. 1A. There was little variation between individual samples in terms of maximum inhibition obtained with native PS. In contrast, greater variability in inhibition was seen after serum samples were incubated with de-O-Ac PS. At 50 μg of inhibiting PS per ml, levels of inhibition ranged from <5 to 70%. Only one serum sample (H-2) of the 18 was substantially (>35%) inhibited from binding to the plate coated with native PS, to a maximum of 75%, by 10 μg of de-O-Ac PS per ml (Fig. 1B).
FIG. 1.
Inhibition of human antibodies binding to N. meningitidis serogroup A capsular PS by native PS (A) and de-O-Ac PS (B). Four representative sera from individuals after vaccination with serogroup A PS vaccine are shown. Fivefold dilutions of inhibiting PS were incubated with a single serum dilution previously shown by ELISA to be in the linear range of the titration curve. Percent inhibition was calculated by comparison of the mean of two OD values for each inhibitor concentration to the mean of two OD values for sera and buffer only.
One serum sample (IJ) that was inhibited by native PS but not de-O-Ac PS was further evaluated to assess the effect of PS polymer size on inhibition. Mild treatment of native PS with HCl for 2 or 4 h generated PSs with average molecular sizes that were less than that of the de-O-Ac PS. Inhibition ELISA was performed using native PS, de-O-Ac PS, and the two partially hydrolyzed O-Ac PSs as inhibitors. Binding of specific antibodies was inhibited by partially hydrolyzed PS less than by native PS but more than by de-O-Ac PS (Fig. 2).
FIG. 2.
Inhibition of serum IJ binding to serogroup A capsular PS by native, de-O-Ac, and size-reduced PS. Note that the polymer length is inversely related to the Kd value.
Murine responses to immunization with native and de-O-Ac PS and conjugate vaccines.
Immunization studies with mice were conducted using native PS, protein-conjugated O-Ac-TT PS, de-O-Ac PS, protein-conjugated de-O-Ac-TT PS, and an adjuvant control. Antibody responses were characterized by ELISA and bactericidal assays. Two independent immunization experiments were conducted.
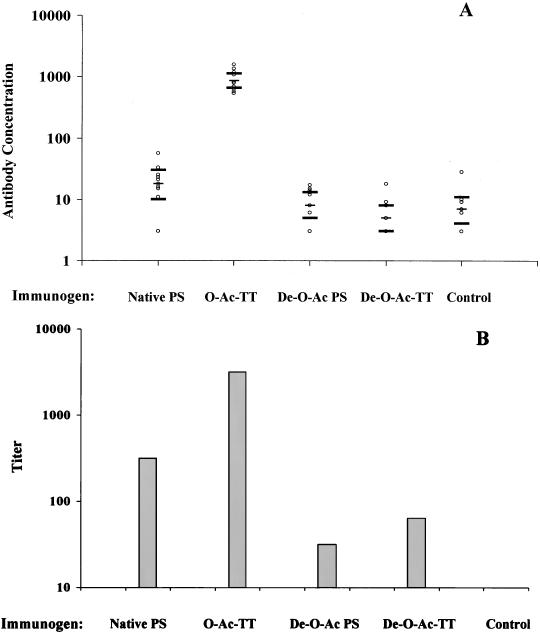
Antibody responses at 4 weeks after the third immunization were determined by PS ELISA. The results from individual sera obtained in experiment 2 are shown in Fig. 3A and are consistent with antibody concentrations measured in pooled sera from experiment 1. Antibody binding to native PS was significantly increased in the native PS- and O-Ac-TT-immunized groups compared to mice immunized with adjuvant alone (control mice), and as expected, the O-Ac-TT conjugate vaccine was more immunogenic than native PS. Antibody levels in the animals immunized with de-O-Ac PS and de-O-Ac-TT conjugate were not significantly different from those in control mice. To determine if de-O-Ac vaccines induced antibodies specific to de-O-Ac PS, ELISAs were also done with native and de-O-Ac PSs conjugated to HSA as coating antigens. Pooled serum from the de-O-Ac-TT-immunized mice bound better than that from the control mice in the de-O-Ac-HSA ELISA (373 and 79 EU, respectively), suggesting a response to immunization. However, even in the de-O-Ac-HSA ELISA, the highest antibody concentration observed was with sera from the O-Ac-TT-immunized mice (991 EU).
FIG. 3.
Antibody responses in mice immunized with 2 μg of native PS, O-Ac-TT, de-O-Ac PS, de-O-Ac-TT, or adjuvant alone (control), as measured by ELISA (A) and by SBA (B). (A) PS ELISA, using native PS mixed with mHSA as the coating antigen, was performed with individual sera. The geometric mean concentration and 95% confidence intervals determined by one-way analysis of variance are shown for each group. Only the geometric mean concentrations in native PS- and O-Ac-TT-immunized animals are significantly different from those in the control mice by Dunnett's test with a significance level of 0.05. (B) The SBA titers shown are for pooled sera for each immunization group. Data from a representative assay are shown; titers obtained from duplicate sera and repeat assays and from an independent immunization experiment were within one twofold dilution for each group.
The level of functional antibody induced in each vaccine group was measured in bactericidal assays (Fig. 3B). The SBA titers in the groups immunized with O-Ac-TT conjugate were more than eightfold higher than those in the group receiving native PS and more than 32-fold higher than those in the group receiving de-O-Ac PS. Unconjugated de-O-Ac PS induced little or no antibody as measured by ELISA; however, measurable SBA titers were seen in this group. Protein conjugation did not substantially increase the level of bactericidal antibody induced by de-O-Ac PS. The highest SBA titer following vaccination with de-O-Ac-TT was achieved with the 2-μg dose and was only a single twofold dilution higher than that with de-O-Ac PS.
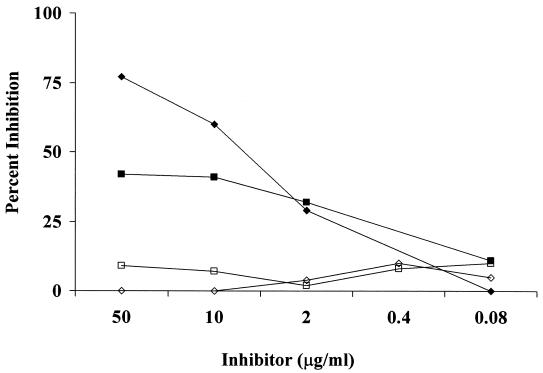
The specificity of mouse antibody responses to vaccination was assessed by using inhibition assays similar to those performed with human immune sera as described above. Substantial inhibition of antibody binding to native PS was seen for sera from both PS- and O-Ac-TT-immunized mice with native, but not de-O-Ac PS, as the inhibitor (Fig. 4).
FIG. 4.
Inhibition of mouse postimmunization sera binding to native PS in the PS ELISA with native and de-O-Ac PS as inhibitors. Anti-serogroup A PS antibodies in sera from mice immunized with PS (diamonds) and O-Ac-TT (squares) are inhibited by native (solid symbols) but not de-O-Ac (open symbols) PS.
Determination of the immunoglobulin subclass also revealed differences between vaccinated groups. The majority of antibodies in the sera obtained from mice immunized with O-Ac-TT conjugate were IgG1, while those in the group immunized with native PS were predominantly IgM and IgG3 (data not shown).
DISCUSSION
The immunologic importance of O-acetyl groups of the N. meningitidis serogroup A PS was demonstrated here by examining human sera from vaccinated individuals and by evaluating the immunogenicities of native and de-O-acetylated PSs in a murine model. The anti-serogroup A antibodies in sera from vaccinated individuals were, in general, specific for O-Ac PS. Gel filtration of the PS samples showed that the size of the de-O-Ac polymer was somewhat reduced by alkaline hydrolysis. Since both the immunogenicity and efficacy of serogroup A PS vaccines are directly related to the molecular size of the PS (8), the possible confounding effect of size reduction on antibody binding to de-O-Ac PS was examined. Native PS samples treated by acid hydrolysis to yield polymers smaller by gel filtration than the de-O-Ac PS were tested as inhibitors. Although the acid-hydrolyzed PS samples demonstrated a reduced ability to inhibit binding of antibody to the plates coated with native PS compared to inhibition with native PS, they were much better inhibitors than the higher-molecular-weight de-O-Ac PS. This indicates that size reduction alone does not account for the differences in inhibition seen between the native and de-O-Ac PSs and suggests that the O-acetyl groups contribute to the immunodominant determinants of serogroup A meningococcal PS.
Since the data from human vaccinee sera indicated that the O-acetyl groups were important in the immune response to serogroup A PS, we compared the O-Ac and the de-O-Ac PS in immunogenicity studies with mice. In these studies, both PS and PS-protein conjugates were used to examine possible differences in the effect of de-O acetylation on immune responses to conjugated or unconjugated PS. In addition, conjugation of PS to protein has been shown to overcome the impact of reduced size on immunogenicity. As expected, PS conjugated to a protein carrier was more immunogenic than the native PS. However, de-O acetylation of serogroup A PS resulted in a marked loss of immunogenicity regardless of protein conjugation. The O-Ac-TT conjugate at all tested doses produced antibody titers of almost 1,000 EU, while the response in animals immunized with the de-O-Ac-TT conjugate was not significantly different from that in control mice. ELISAs, using O-Ac and de-O-Ac PS conjugated to HSA as coating antigens, showed consistent results: the de-O-Ac-TT-immunized mice had much lower levels of serum antibody to either O-Ac or de-O-Ac PS.
Most importantly, the ability to induce functional bactericidal antibody was drastically reduced by de-O acetylation. Both the O-Ac-TT conjugate and native PS induced much higher levels of functional antibody than either conjugated or unconjugated de-O-Ac PS. The de-O-Ac-TT-immunized animals did develop some functional antibody, suggesting that epitopes not involving O-acetyl groups may contribute to the development of a protective response. Additional studies are needed to examine the epitopes involved in producing these antibodies, although further study may be hampered by the overall weak responses seen in de-O-Ac PS-immunized groups.
The antigenic importance of the O-acetyl groups is not surprising given their position on the PS backbone. The O-acetyl groups are bulky, and, as shown for the Vi PS of Salmonella enterica serovar Typhi, they cover most of the reactive moieties of the PS and are readily available to interact with components of the immune response (29). Fattom et al. (7) evaluated the effect of O acetylation of Staphylococcus aureus PS on immunogenicity and found that although the S. aureus PS is heavily O acetylated, stretches of bare PS were able to elicit backbone-specific, bactericidal antibodies. In the present study, only 1 of 18 human sera contained antibodies that bound equally well to both native PS and de-O-Ac PS in the inhibition assay. For this individual, antibodies specific for the PS backbone may be present.
O-acetyl groups have been shown to affect the immunogenicity or antigenicity of several bacterial PSs. Using competitive inhibition ELISA, Arakere et al. (3) evaluated the specificity of antibodies elicited in response to vaccination with an O-acetyl-positive serogroup C N. meningitidis vaccine. Antibodies from vaccinees showed greater affinity for the O-acetyl-positive PS than for the O-acetyl-negative variant. However, the O-acetyl groups on the meningococcal serogroup C PS are not necessary for induction of protective antibodies (26). Similarly, O acetylation of the pneumococcal type 9V PS is not required for induction of opsonic antibodies (18). In contrast, removal of O-acetyl groups from the Vi PS of S. enterica serovar Typhi resulted in complete loss of immunogenicity, and O-acetyl-negative variants of the Escherichia coli K1 capsular PS were less immunogenic than the O-acetyl-positive PS (22, 29). Our studies indicate that the immunogenicity of N. meningitidis serogroup A PS, like that of the Vi PS, depends on the presence of O-acetyl groups.
The bactericidal titers in the groups immunized with unconjugated PS are of interest. The groups that received native PS produced high bactericidal titers relative to ELISA titers. These results may be due to Ig class differences between PS and PS conjugate groups. The relative predominance of IgM and IgG3 seen in the PS group is consistent with previously reported murine responses to PS (21, 28) and may result in more efficient complement fixation and killing. However, the higher proportion of functional antibody induced by unconjugated PS relative to the total anti-PS antibody also raises the concern that the conjugation process affected epitopes of the PS, resulting in relatively high levels of antibodies that are not bactericidal. These data emphasize the need to anticipate and evaluate the effects of conjugation on potential protective epitopes of serogroup A PS and the importance of using an assay that measures functional antibody.
In summary, the vast majority of human antibodies to serogroup A meningococcal PS appear to bind one or more epitopes that are affected when the PS is de-O acetylated. Removal of the O-acetyl groups reduced the immunogenicity of serogroup A PS in mice for both conjugated and unconjugated vaccines. Although mice immunized with native PS produced high bactericidal titers relative to total antibody, immunization with serogroup A PS-TT conjugate resulted in the highest absolute ELISA and bactericidal titers. The de-O-Ac PS was poorly immunogenic, even when conjugated to TT, but induced some bactericidal antibody, suggesting that some epitopes of the de-O-Ac PS are able to induce a weak protective immune response. The results of this study indicate that the O-acetyl groups of serogroup A meningococcal PS are critical to the immunogenicity of the PS. O acetylation is clearly an important parameter to maintain during PS-protein conjugation.
Acknowledgments
We thank Karen Elkins and Martin Ottolini for their thoughtful and critical manuscript review and Alberto Gutierrez for his assistance with proton NMR.
Editor: T. R. Kozel
Footnotes
This paper is dedicated to the memory of Major David Berry, M.D., Medical Corps, U.S. Army, who died in service to his country. Both his friendship and his contribution to the field of pediatric infectious diseases are sorely missed.
REFERENCES
- 1.Achtman, M. 1997. Microevolution and epidemic spread of serogroup A Neisseria meningitidis—a review. Gene 192:135-140. [DOI] [PubMed] [Google Scholar]
- 2.Arakere, G., A. L. Lee, and C. E. Frasch. 1994. Involvement of phospholipid end groups of group C Neisseria meningitidis and Haemophilus influenzae type b polysaccharides in association with isolated outer membranes and in immunoassays. J. Bacteriol. 176:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakere, G., and C. E. Frasch. 1991. Specificity of antibodies to O-acetyl-positive and O-acetyl negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect. Immun. 59:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisgard, K. M., A. Kao, J. Leake, P. M. Strebel, B. A. Perkins, and M. Wharton. 1998. Haemophilus influenzae invasive disease in the United States, 1994-1995: near disappearance of a vaccine-preventable childhood disease. Emerg. Infect. Dis. 4:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constantino, P., S. Viti, A. Podda, M. A. Velmonte, L. Nencione, and R. Rappuoli. 1992. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine 10:691-698. [DOI] [PubMed] [Google Scholar]
- 6.Couchi, S. L., L. E. Markowitz, D. D. Joshi, R. C. Owens, Jr., D. H. Stenhouse, D. N. Regmi, R. P. Shrestha, I. Lacharya, M. Manandhar, and V. L. Gurubacharya. 1987. Control of epidemic group A meningococcal meningitis in Nepal. Int. J. Epidemiol. 16:91-97. [DOI] [PubMed] [Google Scholar]
- 7.Fattom, A. J., J. Sarwar, L. Basham, S. Ennifar, and R. Naso. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasch, C. E. 1995. Meningococcal vaccines: past, present and future, p. 245-283. In K. Cartwright (ed.), Meningococcal disease. John Wiley, New York, N.Y.
- 9.Goldschneider, I., M. L. Lepow, E. C. Gotschlish, F. T. Mauck, F. Bachl, and M. Randolf. 1973. Immunogenicity of group A and group C meningococcal polysaccharides in human infants. J. Infect. Dis. 128:769-776. [DOI] [PubMed] [Google Scholar]
- 10.Jennings, H., A. Bhattacharjee, D. Bundle, C. P. Kenny, A. Martin, and I. C. Smith. 1977. Structure of the capsular polysaccharide of Neisseria meningitidis as determined by 13C-nuclear magnetic resonance spectroscopy. J. Infect. Dis. 136:S78-S83. [DOI] [PubMed] [Google Scholar]
- 11.Kayhty, H., V. Karanko, H. Peltola, S. Sarna, and P. H. Makela. 1980. Serum antibodies to capsular polysaccharide vaccine of group A Neisseria meningitidis followed for three years in infants and children. J. Infect. Dis. 142:861-868. [DOI] [PubMed] [Google Scholar]
- 12.Konadu, E., J. B. Robbins, J. Shiloach, D. A. Bryla, and S. C. Szu. 1994. Preparation, characterization, and immunological properties in mice of Escherichia coli O157 O-specific polysaccharide-protein conjugate vaccines. Infect. Immun. 62:5048-5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leach, A., P. Twumasi, S. Kumah, W. S. Banya, S. Jaffar, B. D. Forrest, D. M. Granoff, D. E. LiButti, G. M. Carlone, L. B. Pais, C. V. Broome, and B. M. Greenwood. 1997. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J. Infect. Dis. 175:200-204. [DOI] [PubMed] [Google Scholar]
- 14.Lemercinier, X., and C. Jones. 1996. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides used in vaccine production. Carbohydr. Res. 296:83-96. [DOI] [PubMed] [Google Scholar]
- 15.Lennon, D., B. Gellin, D. Hood, L. Voss, H. Heffernan, and S. Thakur. 1992. Successful intervention in a group A meningococcal outbreak in Auckland, New Zealand. Pediatr. Infect. Dis. J. 11:617-623. [PubMed] [Google Scholar]
- 16.Lewis, R., N. Nathan, L. Diarra, F. Belanger, and C. Paquet. 2001. Timely detection of meningococcal meningitis in epidemics in Africa. Lancet 358:287-293. [DOI] [PubMed] [Google Scholar]
- 17.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, H. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, and G. M. Carlone. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immun. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeely, T. B., J. M. Staub, C. M. Rusk, M. J. Blum, and J. J. Donnelly. 1998. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect. Immun. 66:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokrenko, A., and V. Kuvakina. 1981. Immunogenicity of serogroup A meningococcal surface polysaccharide with different physicochemical properties. Zh. Mikrobiol. Epidemiol. Immunobiol. 4:59-63. [PubMed] [Google Scholar]
- 20.Monsigny, M., C. Peitt, and A. C. Roche. 1988. Colorimetric determination of neutral sugar by a resorcinol sulphuric acid micromethod. Anal. Biochem. 175:525-530. [DOI] [PubMed] [Google Scholar]
- 21.Muller, E., and M. A. Apicella. 1988. T-cell modulation of the murine antibody response to Neisseria meningitidis group A capsular polysaccharide. Infect. Immun. 56:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orskov, F., I. Orskov, A. Sutton, R. Schneerson, W. Lin, W. Egan, G. E. Hoff, and J. B. Robbins. 1979. Form variation in E. coli K1: determined by O-acetylation of the capsular polysaccharide. J. Exp. Med. 149:669-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinner, R. W., F. Onyango, B. A. Perkins, N. B. Mirza, D. M. Ngacha, M. Reeves, W. DeWitt, E. Njeru, N. N. Agata, and C. V. Broome. 1992. Epidemic meningococcal disease in Nairobi, Kenya, 1989. J. Infect. Dis. 166:359-364. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 25.Reingold, A. L., C. V. Broome, A. W. Hightower, G. W. Ajello, G. A. Bolan, C. Adamsbaum, E. E. Jones, C. Phillips, H. Tiendrebeogo, and A. Yada. 1985. Age-specific differences in duration of clinical protection after vaccination with meningococcal polysaccharide vaccine. Lancet ii:114-118. [DOI] [PubMed]
- 26.Richmond, P., R. Borrow, J. Findlow, S. Martin, C. Thornton, K. Cartwright, and E. Miller. 2001. Evaluation of de-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and de-O-acetylated serogroup C strains. Infect. Immun. 69:2378-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins, J. B., R. Schneerson, and E. C. Gotschlich. 2000. A rebuttal: epidemic and endemic meningococcal meningitis in sub-Saharan Africa can be prevented now by routine immunization with group A meningococcal capsular polysaccharide vaccine. Pediatr. Infect. Dis. J. 19:945-953. [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein, L. J., P. A. Garcia-Ojeda, F. Michon, H. J. Jennings, and K. E. Stein. 1998. Murine immune responses to Neisseria meningitidis group C capsular polysaccharide and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 66:5450-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szu, S., X. R. Li, A. L. Stone, and J. B. Robbins. 1991. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 59:4555-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twumasi, P., S. Kumah, A. Leach, T. J. O'Dempsey, S. J. Ceesay, J. Todd, C. V. Broome, G. M. Carlone, L. B. Pais, P. K. Holder, et al. 1995. A trial of group A plus group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J. Infect. Dis. 171:632-638. [DOI] [PubMed] [Google Scholar]
- 31.Wong, K. H., O. Barrera, A. Sutton, J. May, D. H. Hochstein, J. D. Robbins, J. B. Robbins, P. D. Parkman, and E. B. Seligmann, Jr. 1977. Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J. Biol. Stand. 5:197-215. [DOI] [PubMed] [Google Scholar]
- 32.Zangwill, K. M., R. W. Stout, G. M. Carlone, L. Pais, H. Harekeh, S. Mitchell, W. H. Wolfe, V. Blackwood, B. D. Plikaytis, and J. D. Wenger. 1994. Duration of antibody response after meningococcal polysaccharide vaccination in U.S. Air Force personnel. J. Infect. Dis. 169:847-852. [DOI] [PubMed] [Google Scholar]