Abstract
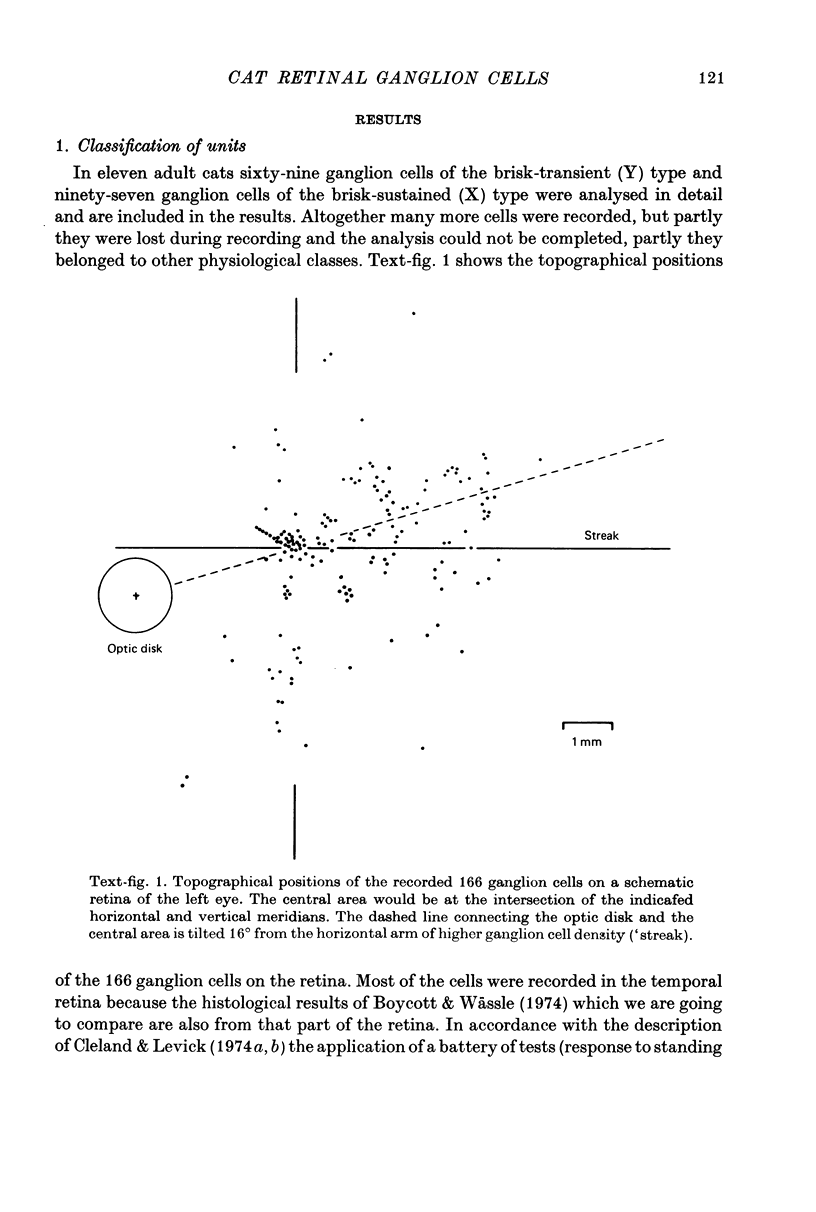
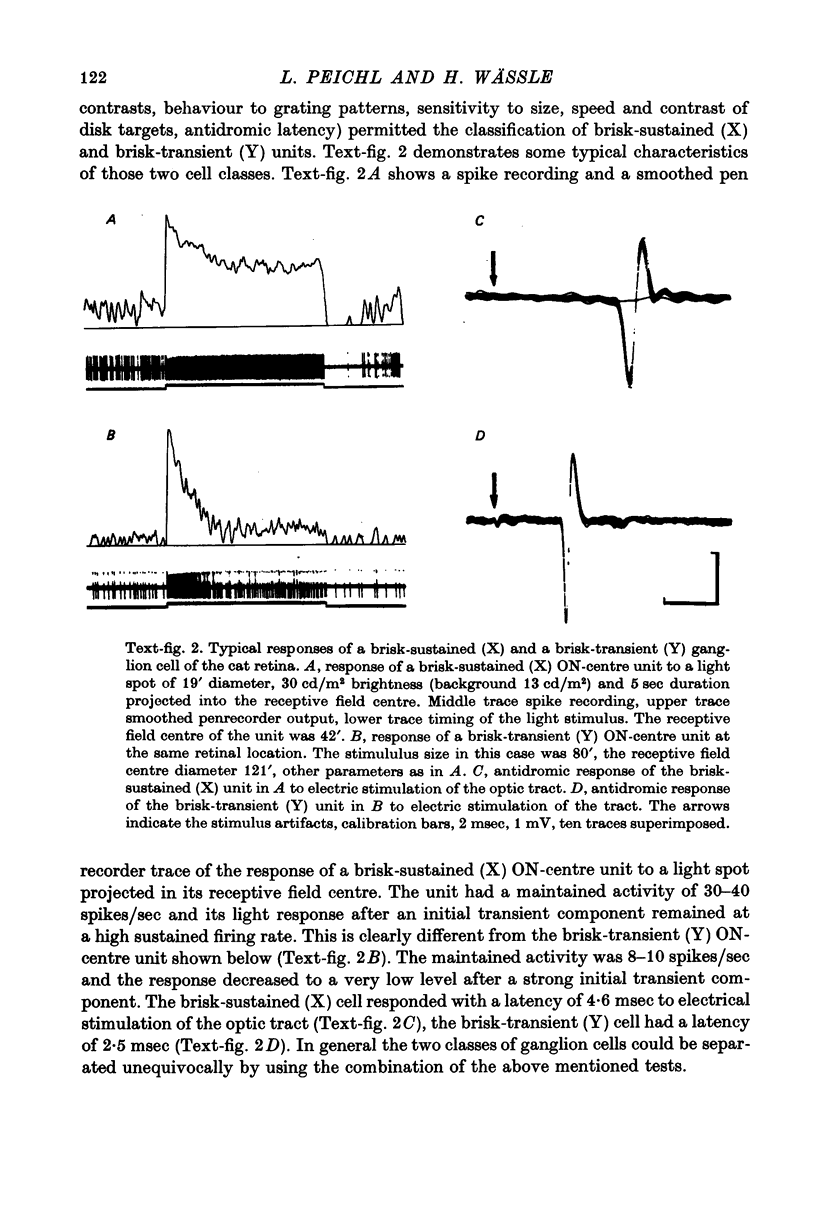
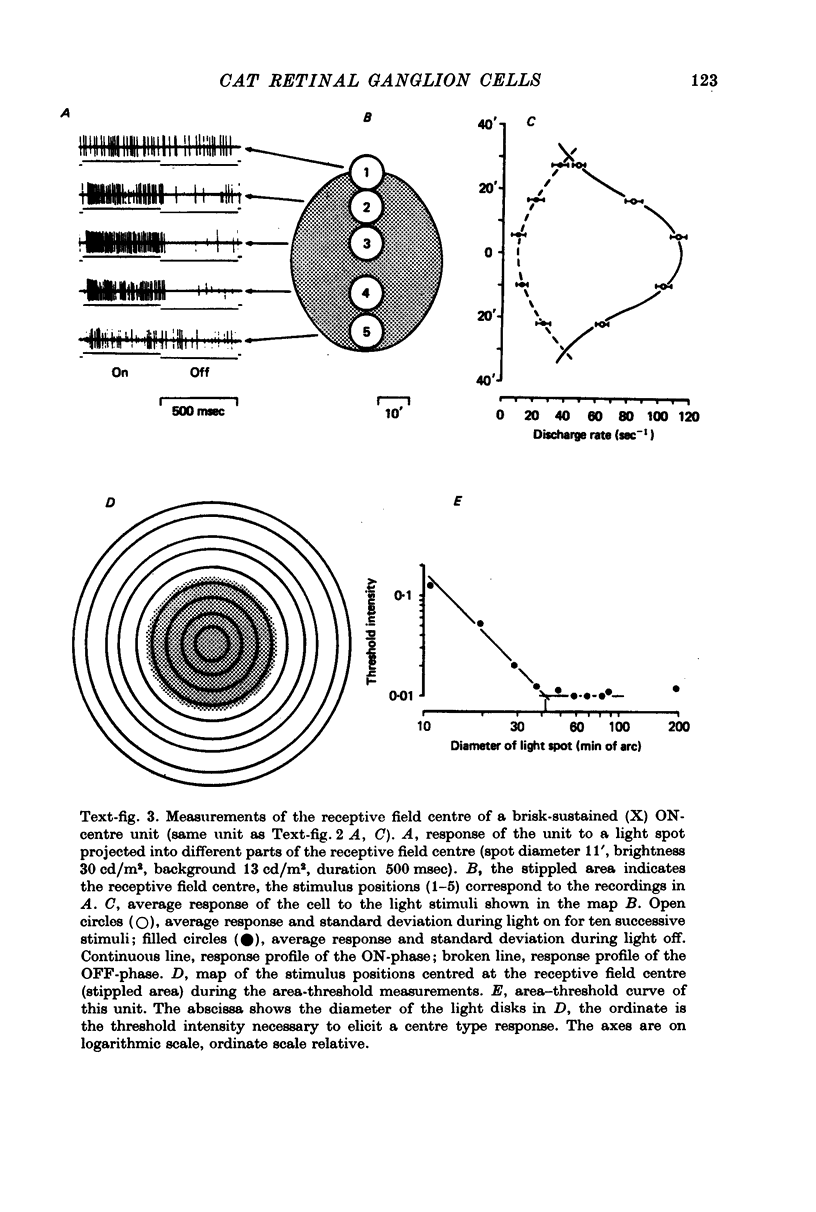
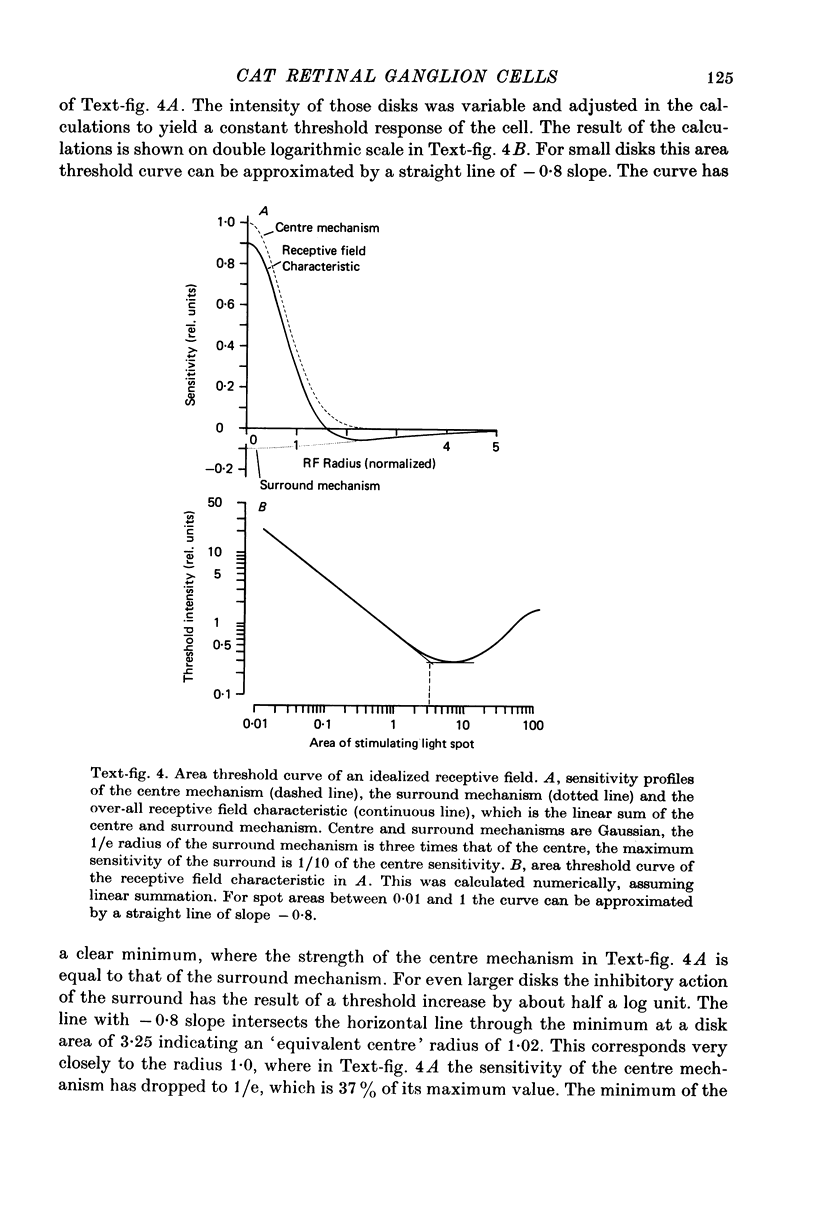
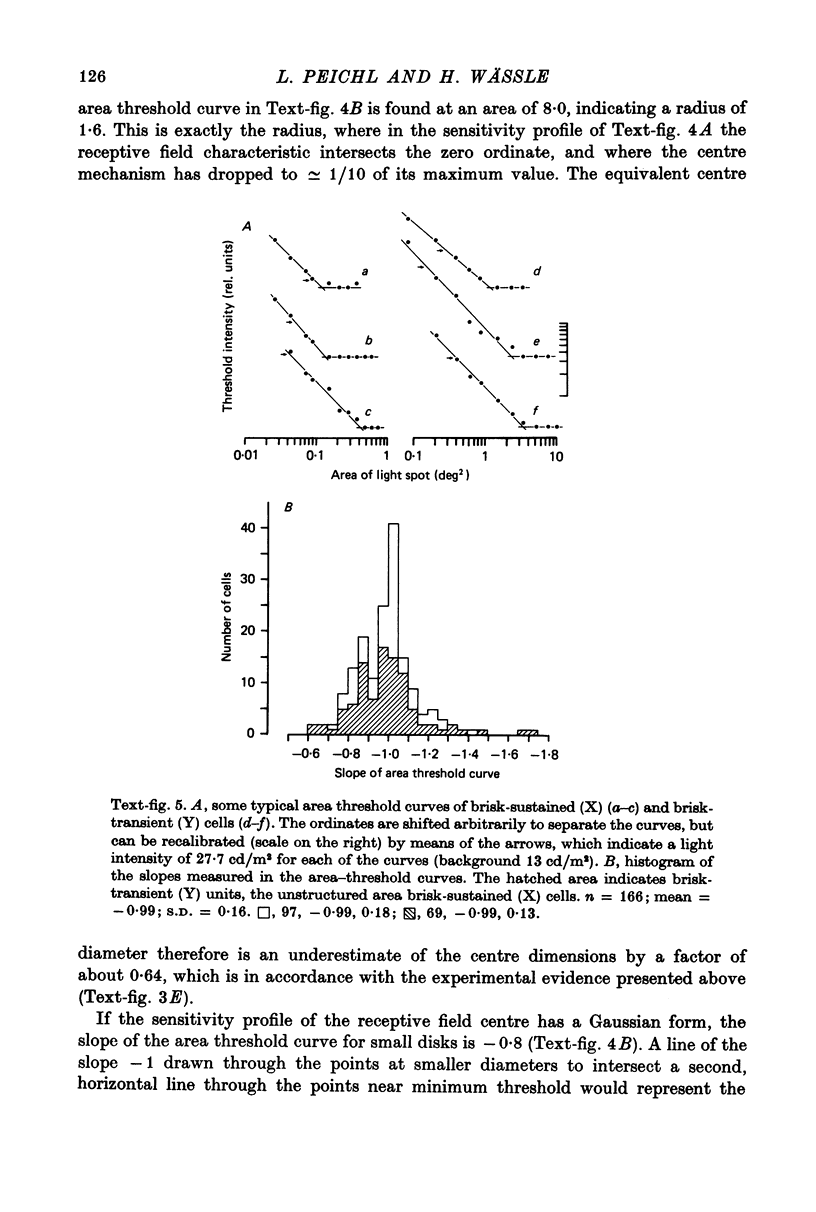
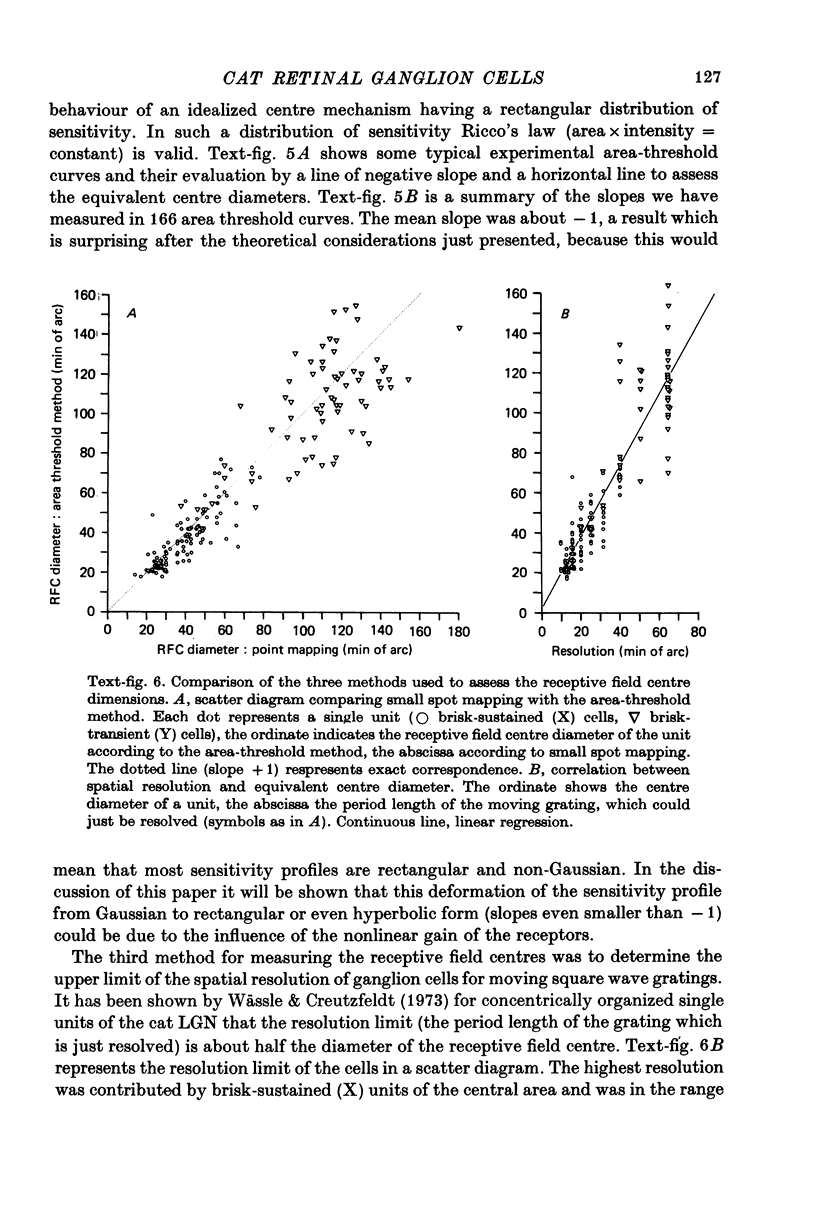
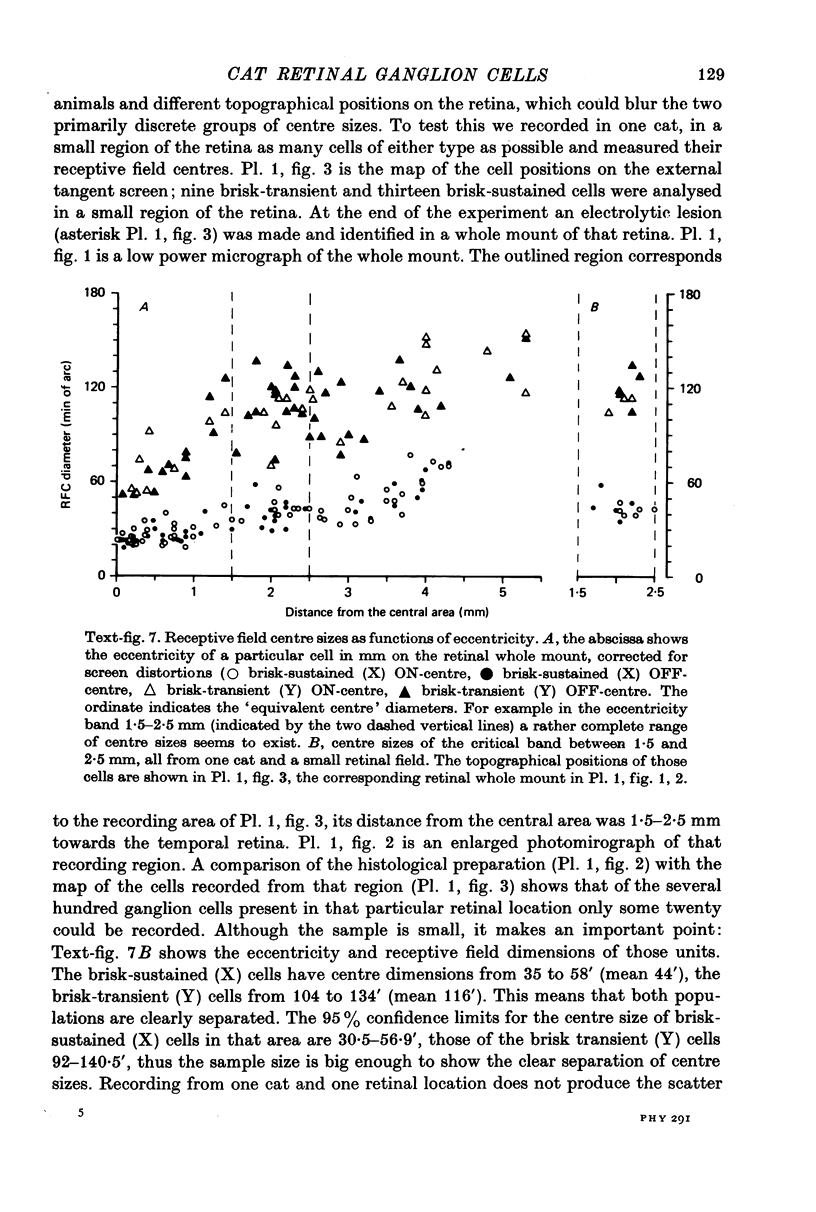
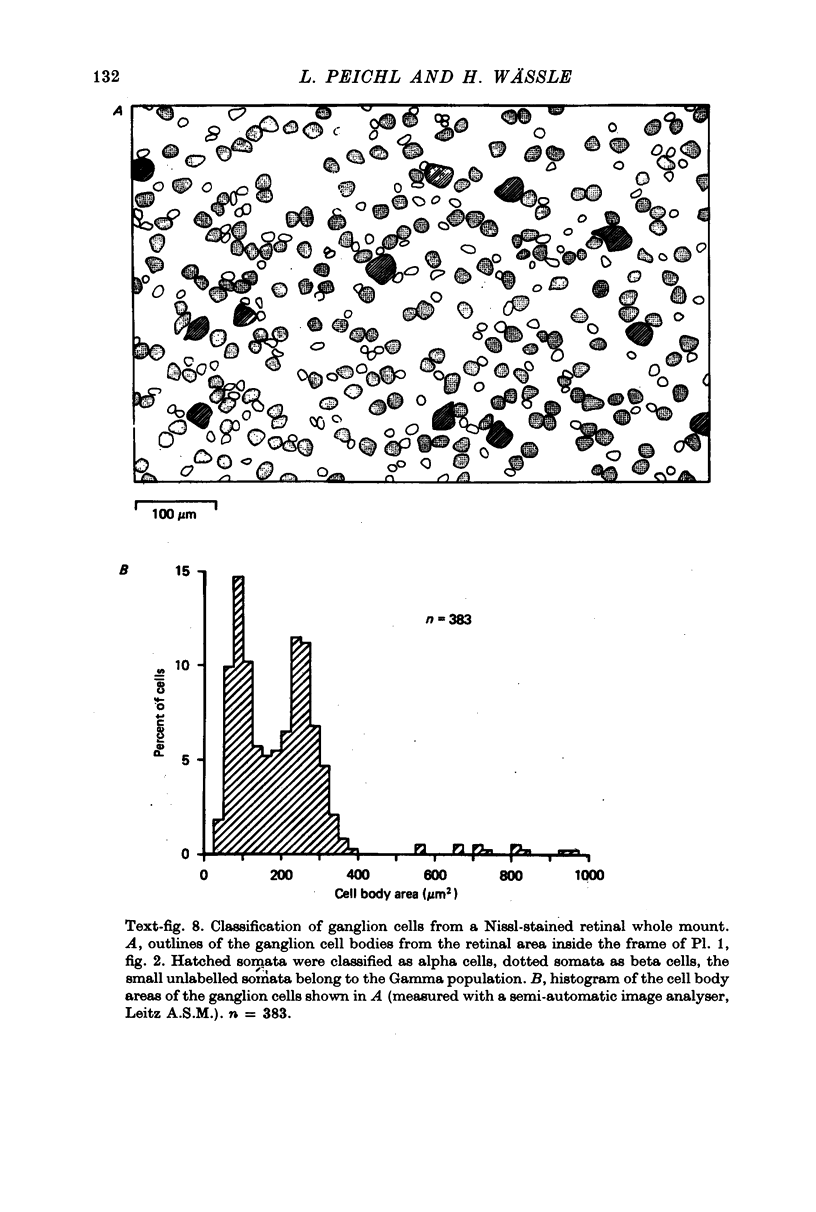
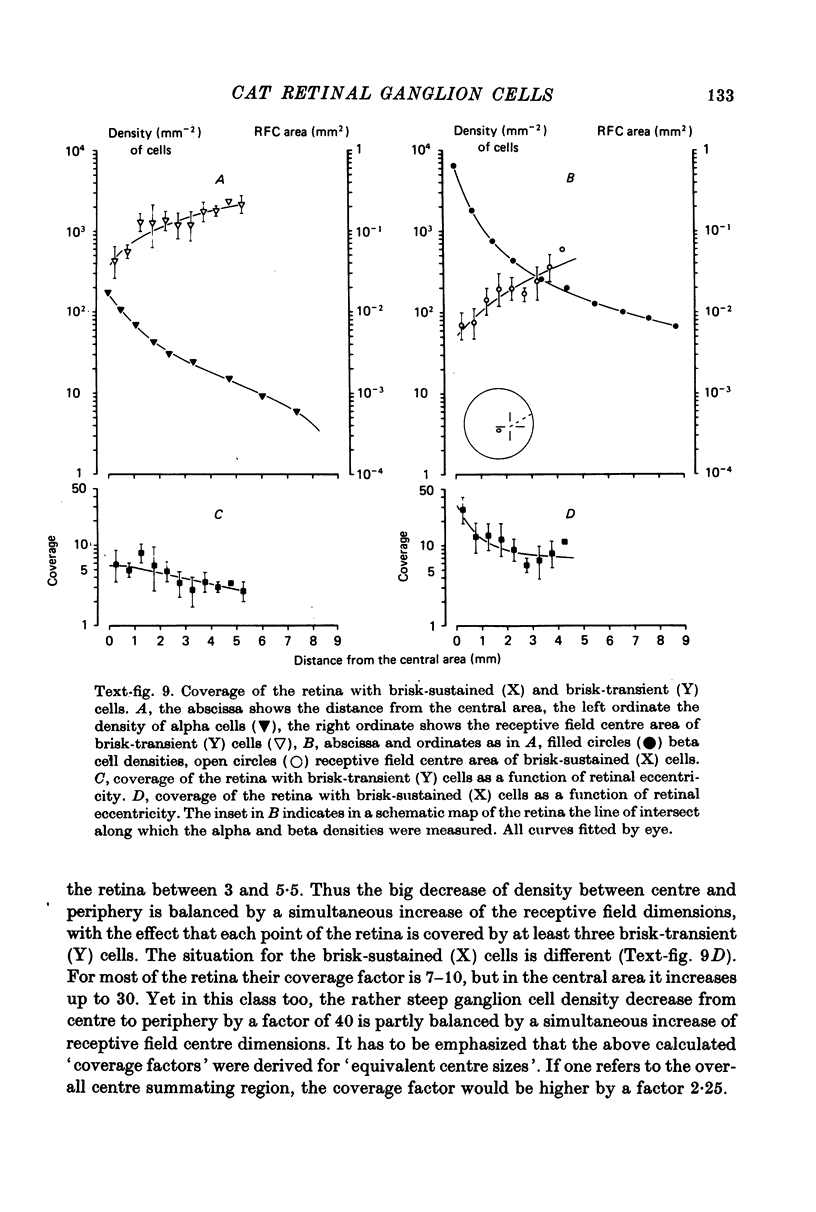
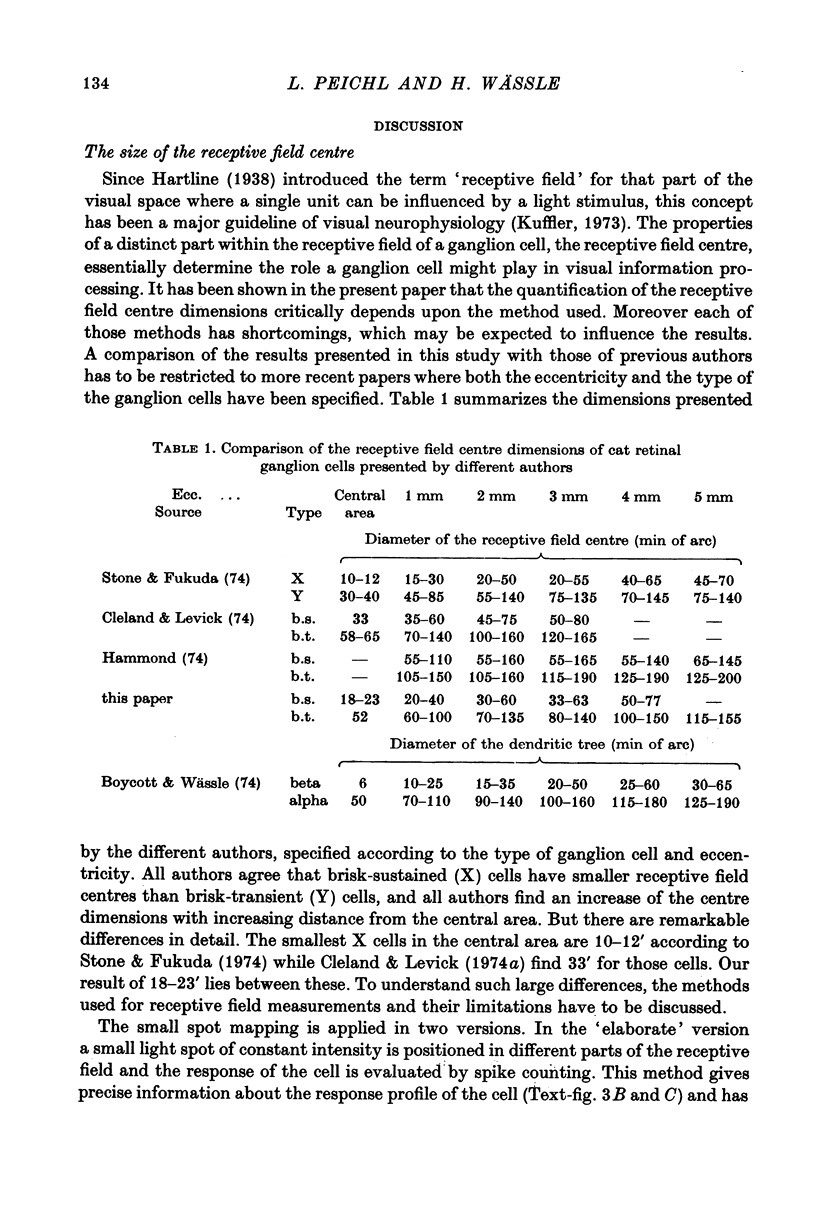
1. Receptive field centre sizes of brisk-sustained (X) and brisk-transient (Y) ganglion cells of the cat retina were assessed by three different methods: small spot mapping, area threshold method and spatial resolution. 2. Centre sizes of brisk-sustained (X) cells increased from 20' in the central area to about 70' at an eccentricity of 4.5 mm, centre sizes of brisk-transient (Y) cells from 50' in the central area to about 140' at 5 mm eccentricity. 3. The scatter of centre sizes at one retinal location was measured by recording as many ganglion cells as possible in one cat in a small field of retina. The centre sizes of the individual classes were homogeneous and exhibited only a small amount of scatter. 4. The coverage of the retina by the different ganglion cell classes was assessed from their density and their receptive field centre area. At every retinal location the receptive field centres of seven to twenty brisk-sustained (X) cells and of three to six brisk-transient (Y) cells were found to overlap. Sluggish concentric and non-concentric cells taken together have a coverage factor of about 60.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Exp Brain Res. 1975 Dec 22;24(2):159–179. doi: 10.1007/BF00234061. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Peichl L., Wässle H. Morphological types of horizontal cell in the retina of the domestic cat. Proc R Soc Lond B Biol Sci. 1978 Dec 18;203(1152):229–245. doi: 10.1098/rspb.1978.0103. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Major D. Cat retinal ganglion cell dendritic fields. Exp Neurol. 1966 May;15(1):70–78. doi: 10.1016/0014-4886(66)90035-5. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Wässle H. Physiological identification of a morphological class of cat retinal ganglion cells. J Physiol. 1975 Jun;248(1):151–171. doi: 10.1113/jphysiol.1975.sp010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Sakmann B., Scheich H., Korn A. Sensitivity distribution and spatial summation within receptive-field center of retinal on-center ganglion cells and transfer function of the retina. J Neurophysiol. 1970 Sep;33(5):654–671. doi: 10.1152/jn.1970.33.5.654. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz B. G., Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):297–318. doi: 10.1113/jphysiol.1977.sp011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- Fischer B., Krüger J., Droll W. Quantitative aspects of the shift-effect in cat retinal ganglion cells. Brain Res. 1975 Jan 17;83(3):391–403. doi: 10.1016/0006-8993(75)90832-x. [DOI] [PubMed] [Google Scholar]
- Fischer B. Overlap of receptive field centers and representation of the visual field in the cat's optic tract. Vision Res. 1973 Nov;13(11):2113–2120. doi: 10.1016/0042-6989(73)90188-0. [DOI] [PubMed] [Google Scholar]
- Graham N., Robson J. G., Nachmias J. Grating summation in fovea and periphery. Vision Res. 1978;18(7):815–825. doi: 10.1016/0042-6989(78)90122-0. [DOI] [PubMed] [Google Scholar]
- Hammond P. Cat retinal ganglion cells: size and shape of receptive field centres. J Physiol. 1974 Oct;242(1):99–118. doi: 10.1113/jphysiol.1974.sp010696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Hughes A. A quantitative analysis of the cat retinal ganglion cell topography. J Comp Neurol. 1975 Sep;163(1):107–128. doi: 10.1002/cne.901630107. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W. The single-cell approach in the visual system and the study of receptive fields. Invest Ophthalmol. 1973 Nov;12(11):794–813. [PubMed] [Google Scholar]
- LEVICK W. R., OYSTER C. W., DAVIS D. L. EVIDENCE THAT MCILWAIN'S PERIPHERY EFFECT IS NOT A STRAY LIGHT ARTIFACT. J Neurophysiol. 1965 May;28:555–559. doi: 10.1152/jn.1965.28.3.555. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Form and function of cat retinal ganglion cells. Nature. 1975 Apr 24;254(5502):659–662. doi: 10.1038/254659a0. [DOI] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Snyder A. W., Miller W. H. Photoreceptor diameter and spacing for highest resolving power. J Opt Soc Am. 1977 May;67(5):696–698. doi: 10.1364/josa.67.000696. [DOI] [PubMed] [Google Scholar]
- Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol. 1965 Jun;124(3):337–352. doi: 10.1002/cne.901240305. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffmann K. P. Very slow-conducting ganglion cells in the cat's retina: a major, new functional type? Brain Res. 1972 Aug 25;43(2):610–616. doi: 10.1016/0006-8993(72)90416-7. [DOI] [PubMed] [Google Scholar]
- Venes J. L., Collins W. F., Taub A. Nitrous oxide: an anesthetic for experiments in cats. Am J Physiol. 1971 Jun;220(6):2028–2031. doi: 10.1152/ajplegacy.1971.220.6.2028. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. A stereotaxic headholder for visual neurophysiology. Exp Brain Res. 1975 Aug 14;23(2):151–156. doi: 10.1007/BF00235457. [DOI] [PubMed] [Google Scholar]
- Wässle H., Boycott B. B., Peichl L. Receptor contacts of horizontal cells in the retina of the domestic cat. Proc R Soc Lond B Biol Sci. 1978 Dec 18;203(1152):247–267. doi: 10.1098/rspb.1978.0104. [DOI] [PubMed] [Google Scholar]
- Wässle H., Creutzfeldt O. D. Spatial resolution in visual system: a theoretical and experimental study on single units in the cat's lateral geniculate body. J Neurophysiol. 1973 Jan;36(1):13–27. doi: 10.1152/jn.1973.36.1.13. [DOI] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. Topography of horizontal cells in the retina of the domestic cat. Proc R Soc Lond B Biol Sci. 1978 Dec 18;203(1152):269–291. doi: 10.1098/rspb.1978.0105. [DOI] [PubMed] [Google Scholar]