Abstract
Immunological and epidemiological evidence suggests that the development of natural immunity to meningococcal disease results from colonization of the nasopharynx by commensal Neisseria spp., particularly with N. lactamica. We report here that immunization with N. lactamica killed whole cells, outer membrane vesicles, or outer membrane protein (OMP) pools and protected mice against lethal challenge by a number of diverse serogroup B and C meningococcal isolates in a model of bacteremic infection. Sera raised to N. lactamica killed whole cells, OMPs, or protein pools were found to cross-react with meningococcal isolates of a diverse range of genotypes and phenotypes. The results confirm the potential of N. lactamica to form the basis of a vaccine against meningococcal disease.
Invasive disease caused by Neisseria meningitidis represents a significant global public health problem, and its prevention by vaccination remains a high priority in the United Kingdom and worldwide. In England and Wales, as in many temperate countries, the age-specific incidence of meningococcal disease is highest among infants between the ages of 3 and 12 months (15). Until recently in England and Wales, serogroup B strains accounted for over 50% of cases, and serogroup C strains accounted for 30% (16). However, since the introduction of the conjugated meningococcal serogroup C vaccine into the United Kingdom in late 1999, cases of serogroup C disease have fallen by about 75% in the first age groups targeted to receive this vaccine (22, 24).
Given the propensity of virulent clones of meningococci to switch serogroups by the exchange of genes encoding their capsular polysaccharide (27) or by serogroup replacement through immune selection, there is a concern that a decrease in serogroup C infection might be accompanied by an increase in serogroup B infection (20). This has emphasized the urgent need for comprehensive vaccines offering protection against all virulent meningococci.
Most meningococcal infections are asymptomatic, and almost all individuals are likely to carry meningococci in the nasopharynx at some time during their life. Protection against invasive meningococcal disease has been associated with the presence, in serum, of antibodies which are bactericidal against N. meningitidis, with immune lysis the likely effector mechanism (11). These antibodies are acquired progressively over the first few years of life (11), a period when meningococcal carriage rates are low (6). It has been postulated that acquisition of natural immunity in young children may follow colonization by nonpathogenic species of Neisseria and some other bacteria expressing immunologically cross-reactive surface antigens shared with meningococci (13). Epidemiological evidence suggests that N. lactamica, a commensal species that does not possess a polysaccharide capsule and has common antigens with N. meningitidis (4, 17), may be the most important of these.
N. lactamica exhibits a higher force of infection in young children than does N. meningitidis, passing more freely from child to child, with a shorter duration of carriage (10). The peak carriage rate (approximately 20%) for N. lactamica is in the 0- to 4-year age group (6, 10), which appears to precede a reduced incidence of meningococcal disease in older children (10, 11). In a longitudinal study, Gold et al. (10) demonstrated that 66% of the N. lactamica carriers studied developed antibodies that were bactericidal for meningococci of serogroups A, B, and C. These investigators went on to suggest that induction of such antibodies contributed to the age-related development of natural immunity to meningococcal disease. This hypothesis was supported by the findings of a study conducted in the Faroe Islands (23), which showed that a high prevalence of N. lactamica carriage was associated with a reduced incidence of meningococcal disease. Recent mathematical modeling studies have further supported this finding (7). N. lactamica appears to behave as an almost perfect commensal and has only very rarely been associated with invasive disease (29).
The present study explores experimentally the hypothesis that immunization with N. lactamica can mimic infection by and enhance natural immunity to the meningococcus. We have investigated the cross-reactive antibody response elicited by antigens of N. lactamica to the meningococcus and the potential of these antigens for the development of a comprehensive vaccine against meningococcal disease.
MATERIALS AND METHODS
Bacterial isolates and growth media.
N. lactamica Y92-1009 was obtained from the Meningococcal Reference Unit (Manchester Public Health Laboratory) and was originally isolated during a school carriage study in Northern Ireland. The isolates of N. meningitidis used in this study were isolated from the blood or cerebrospinal fluid of meningococcal disease patients. Isolates K454 and MC58 (B:15:P1.7,16) were originally obtained during a prolonged outbreak of meningococcal disease in Gloucestershire, United Kingdom (5). Isolate B16B6 (B:2a:P1.2) was described by Frasch et al. (9), and GN (C:NT:NST) was described by Ala'Aldeen et al. (2). L91-543 (C:2a:P1.2) was originally isolated from the cerebrospinal fluid of a 14-year-old patient. NZ394/98 was obtained from D. Martin, Communicable Disease Center, Porirua, New Zealand. Other meningococcal isolates were obtained from M. Achtman, Max Planck Institute, Berlin, Germany (Table 1).
TABLE 1.
N. meningitidis and N. lactamica strains used for preparation of vaccines, bactericidal assay target strains, ELISA, and challenge
| Species | Strain | Sequence type | Notes |
|---|---|---|---|
| N. meningitidis | Z3524 | ST5 | A, subgroup III, Chad |
| BZ10 | ST8 | B, A4 cluster, Z4662, The Netherlands | |
| FAM18 | ST11 | C, ET-37 complex, Z8948, USA | |
| ROU | ST11 | W135, ET-37 complex, Z6904, France | |
| L91-543 | ST11 | C2a:P1.2, ET-37 complex, UK | |
| B16B6 | ST11 | B2a:P1.2, ET-37 complex, USA | |
| Z7990 | ST25 | B, Z7990, Norway | |
| BZ198 | ST41 | B nontypeable, lineage 3, Z4673, The Netherlands | |
| NZ394/98 | ST42 | B4:P1.4, lineage 3, New Zealand | |
| BZ147 | ST48 | B:NT, The Netherlands | |
| K454 | ST74 | B15:P1.7,16, ET-5, UK | |
| MC58 | ST74 | B15:P1.7,16, ET-5, UK | |
| C11 | ST345 | C, UK | |
| GN | ST1604 | C nontypeable, UK | |
| N. lactamica | Y92-1009 | UK |
All bacterial stocks were maintained at −70°C in Mueller-Hinton broth (MHB; Oxoid) containing 30% (vol/vol) glycerol. N. lactamica and N. meningitidis were cultured on blood agar (BA) containing 7% (vol/vol) horse blood or in MHB. Cultures on BA were incubated overnight at 37°C in the presence of 5% CO2. MHB was incubated at 37°C with shaking. Iron-limited growth was achieved by the addition of 5 μg of ethylenediamine dihydroxyphenylacetic acid (EDDHA) ml−1 to MHB.
Preparation of OMVs.
N. lactamica Y92-1009 and N. meningitidis K454 outer membrane vesicles (OMVs) were prepared from overnight culture in MHB containing 5 μg of EDDHA ml−1 with lithium acetate extraction (2).
Purification of LOS.
Lipooligosaccharide (LOS) was prepared according to the method described by Gu and Tsai (14).
Separation of N. lactamica proteins by preparative electrophoresis.
N. lactamica cells grown in MHB plus EDDHA were resuspended in 0.3% (vol/vol) Elugent detergent (Calbiochem) in phosphate-buffered saline (PBS) for 20 min at 20°C, and the bacteria were removed by centrifugation (25,000 × g for 10 min at 4°C). The detergent extract supernatant (DE) was then fractionated with a model 491 Prep Cell (Bio-Rad) with a 7% (wt/vol) acrylamide resolving gel and a 4% (wt/vol) acrylamide stacking gel. Samples were applied to the gel in nondenaturing sample buffer (0.5 M Tris, 14% [vol/vol] glycerol) with 0.2 M glycine-25 mM Tris-3.5 mM sodium dodecyl sulfate (SDS) (pH 8.3) as the resolving buffer. Fractions were eluted in the same buffer without SDS and glycine, and pools of proteins were made on the basis of apparent molecular weight, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Protein determination.
Protein concentrations were determined with the bicinchoninic acid protein assay (Pierce).
Immunizations.
Vaccines were prepared with aluminum hydroxide (Alhydrogel; Superfos Biosector; final concentration, 4 mg ml−1) or an equal volume of either Freund's complete adjuvant (first immunization) or Freund's incomplete adjuvant (subsequent immunizations). NIH mice (6 to 8 weeks old) (Harlan) were immunized on days 1, 21, and 28. Each mouse received 10 μg of protein in 0.2 ml by subcutaneous injection. Killed whole-cell vaccines were prepared with N. lactamica grown in MHB plus EDDHA. Cultures were harvested by centrifugation and resuspended to an absorbance of 0.1 (600 nm) in PBS containing 0.1% (wt/vol) thimerosal and 0.1% (vol/vol) formaldehyde. Following confirmation of sterility, the suspension was washed with PBS and diluted to the starting volume in PBS and Alhydrogel, to give a final concentration of 4 mg of aluminum hydroxide ml−1.
Terminal sera were collected on day 35, and for protection studies mice were challenged on day 35.
Infection of mice with N. meningitidis.
Mice were infected by intraperitoneal injection of N. meningitidis (12, 28). Iron-limited bacteria were grown in MHB supplemented with EDDHA for 4 h, adjusted to the required density with the same medium, and mixed with an equal volume of sterile human transferrin (40 mg ml−1; Sigma) in PBS. Mice received the appropriate challenge dose in a total volume of 0.5 ml, and 24 h later a second intraperitoneal injection of 0.2 ml of saline containing human transferrin (50 mg ml−1) was administered. Signs of ill health were monitored for up to 70 h postinfection, and mice were sacrificed before the severity limit of the experiment was exceeded. The virulence of each meningococcal strain was determined following a virulence titration in unvaccinated mice. Two challenge doses were then chosen, a dose sufficient to leave no survivors and a dose approximately 200 times greater than that.
All procedures involving animals were conducted according to the requirements of the United Kingdom Home Office Animals (Scientific Procedures) Acts, 1986.
Whole-cell ELISA.
A whole-cell enzyme-linked immunosorbent assay (ELISA) was performed as described previously (1). Briefly, the bacteria (Table 2) were grown in MHB (Oxoid), made iron limited by the addition of 5 μg of EDDHA ml−1, for 6 h. Suspensions of bacteria were prepared to an optical density at 650 nm of 0.1 in PBS, and the bacteria were then heat killed by incubation at 56°C for 30 min. A heat-killed suspension of Escherichia coli DH5α was prepared as a negative control. The heat-killed suspensions were used to coat 96-well microtiter plates (Nunc Maxisorp) by adding 100 μl per well, and the plates were allowed to dry at 37°C for 48 h. Specific antibodies to whole cells were detected with goat anti-mouse immunoglobulin G-horseradish peroxidase conjugate (ICN) and TMBlue (Universal Biologicals). The antibody titer was defined as the reciprocal dilution of serum corresponding to the midpoint of the dose-response curve; this was calculated with interpolation software (Genesis; Labsystems) on dose-response curves generated from eight dilutions of each serum (1:100 through seven further threefold dilutions). Interplate variation was corrected for by using a pool of serum standards. Each mouse serum was assayed in duplicate, and the mean of the duplicate titers from each of five different mice was used to generate a geometric mean titer for the group (Table 2).
TABLE 2.
Whole-cell ELISA showing cross-reactivity of N. lactamica and N. meningitidis OMV mouse sera against a diverse panel of meningococcal strains
| Strain | Log whole-cell ELISA IgG titer in OMV immunization groupa
|
|
|---|---|---|
| N. lactamica OMV | N. meningitidis OMV | |
| N. lactamica Y92-1009 | 4.84 | 4.13 |
| N. meningitidis | ||
| BZ147 | 4.25 | 4.10 |
| ROU | 5.39 | 4.04 |
| Z3524 | 4.25 | 4.93 |
| NZ 394/98 | 4.36 | 4.03 |
| K454 | 4.45 | 4.79 |
| FAM18 | 4.47 | 4.21 |
| MC58 | 4.19 | 4.48 |
| L91-543 | 4.90 | 4.65 |
| BZ198 | 4.30 | 4.02 |
| B16B6 | 4.39 | 4.63 |
| GN | 4.68 | 4.15 |
| Z7990 | 4.28 | 4.21 |
| E. coli DH5α | <2.00 | <2.00 |
Geometric mean of the titer from five separate animals.
Serum bactericidal assays.
Serum bactericidal assays were performed according to standardized methodology (Centers for Disease Control, Report of the 2nd International Workshop on Meningococcal Immunology and Serology, 1992). Target strains were grown as single colonies on blood agar plates overnight at 37°C in 5% CO2. Approximately 10 colonies were used to inoculate fresh agar plates and incubated for 4 h. Bacteria from these plates were then transferred to 5 ml of bactericidal buffer (0.5% [wt/vol] bovine serum albumin in Gey's balanced salt solution) and further diluted to give a working concentration of 8 × 104 CFU ml−1. The complement source was either 25% (vol/vol) baby rabbit serum (Pelfreeze), for serogroup C strains, or human serum previously screened for lack of antibodies to meningococcal whole cells (by ELISA), for serogroup B strains. Titers are expressed as the reciprocal of the final dilution giving ≥50% bactericidal killing at 60 min. Each assay included control sera comprising pooled unvaccinated mouse sera and monoclonal antibodies against either serogroup B or C polysaccharide (obtained from the National Institute for Biological Standards and Control, United Kingdom).
RESULTS
Cross-reactivity of antisera raised against Neisseria OMVs with N. meningitidis.
Mouse sera raised against N. lactamica Y92-1009 or N. meningitidis K454 OMVs were assessed for cross-reactivity with a variety of N. meningitidis isolates of a range of serogroups, serotypes, and serosubtypes in a whole-cell ELISA. N. lactamica and N. meningitidis OMV sera elicited comparable antibody titers against the strains (Table 2). The bactericidal activity of each serum was assessed against the meningococcal strains Z3524, 394/98, BZ198, BZ10, K454, FAM18, and C11. Bactericidal activity was not detected in sera raised against N. lactamica OMVs. Only mouse serum raised against N. meningitidis OMVs showed in vitro bactericidal activity toward these strains (data not shown).
Immunization with N. lactamica protects mice against experimental infection with N. meningitidis serogroup B.
Groups of five mice were immunized with N. lactamica whole cells, OMVs, or purified LOS and then challenged with 108 CFU of N. meningitidis strain K454. The results in Table 3 show that the progression of disease in unimmunized mice was rapid, with no survivors 24 h postchallenge. All mice immunized with N. lactamica whole cells or OMVs survived this meningococcal challenge (Table 3); although animals in these groups showed mild symptoms of infection at 24 h, they were fully recovered 52 h after infection. In contrast, vaccination with purified N. lactamica LOS afforded no protection against this meningococcal challenge.
TABLE 3.
Immunization of mice with N. lactamica whole cells or OMVs protects against intraperitoneal challenge with N. meningitidis K454a
| Vaccine | No. of survivors (no. healthy)
|
No. of survivors/ no. in group at 72 h | |
|---|---|---|---|
| 24 h | 52 h | ||
| None | 0 | 0 | 0/5 |
| Killed whole cells | 5 (0) | 5 (5) | 5/5 |
| OMVs | 5 (0) | 5 (5) | 5/5 |
| Purified LOS | 3 (0) | 0 | 0/5 |
The challenge dose was 108 CFU of K454. Whole cells were prepared with Alhydrogel; OMVs and LOS were prepared in Freund's complete adjuvant (first immunization) or Freund's incomplete adjuvant (subsequent immunizations).
Protection by N. lactamica or N. meningitidis OMVs against experimental infection with different isolates of N. meningitidis.
The protective efficacy of OMVs prepared from N. lactamica Y92-1009 and N. meningitidis K454 were compared in the mouse infection model with five meningococcal challenge strains (K454, BZ198, FAM18, NZ394/98, and BZ10) representing different clonal lineages (Table 4). Immunization with N. lactamica OMVs protected mice against challenge with all of the meningococcal strains tested. With all of the challenge strains except the homologous N. meningitidis strain, the protection afforded by N. lactamica OMVs compared well with that conferred by the meningococcal OMVs. With some strains, a delay in time to death was observed in mice immunized with N. lactamica OMVs, although the outcome was similar to that observed following immunization with meningococcal OMVs.
TABLE 4.
Immunization of mice with N. lactamica or N. meningitidis OMVs protects against challenge with diverse meningococcal strains of different clonal lineages
| N. meningitidis challenge strain | Challenge dose (CFU) | Vaccinea | No. of survivors (no. healthy)
|
No. of survivors/ no. in group at 72 h | |
|---|---|---|---|---|---|
| 24 h | 52 h | ||||
| K454 | 6 × 105 | None | 0 | 0 | 0/5 |
| NL OMVs | 5 (5) | 5 (5) | 5/5 | ||
| NM OMVs | 5 (5) | 5 (5) | 5/5 | ||
| 6 × 108 | None | 0 | 0 | 0/5 | |
| NL OMVs | 0 | 0 | 0/5 | ||
| NM OMVs | 4 (3) | 3 (3) | 3/5 | ||
| BZ198 | 2 × 106 | None | 3 (0) | 3 (3) | 3/5 |
| NL OMVs | 5 (4) | 5 (5) | 5/5 | ||
| NM OMVs | 5 (5) | 5 (5) | 5/5 | ||
| 2.7 × 107 | None | 0 | 0 | 0/5 | |
| NL OMVs | 4 (4) | 4 (4) | 4/5 | ||
| NM OMVs | 2 (2) | 2 (2) | 2/5 | ||
| FAM18 | 1.6 × 106 | None | 0 | 0 | 0/5 |
| NL OMVs | 4 (4) | 4 (4) | 4/5 | ||
| NM OMVs | 3 (3) | 3 (3) | 3/5 | ||
| 2.3 × 106 | None | 0 | 0 | 0/5 | |
| NL OMVs | 2 (2) | 2 (2) | 2/5 | ||
| NM OMVs | 2 (2) | 2 (2) | 2/5 | ||
| NZ 394/98 | 5.5 × 106 | None | 5 (1) | 1 (1) | 1/5 |
| NL OMVs | 5 (3) | 5 (5) | 5/5 | ||
| NM OMVs | 5 (2) | 5 (5) | 5/5 | ||
| 7.5 × 107 | None | 0 | 0 | 0/5 | |
| NL OMVs | 5 (3) | 4 (4) | 4/5 | ||
| NM OMVs | 5 (5) | 5 (5) | 5/5 | ||
| BZ10 | 1.6 × 106 | None | 0 | 0 | 0/5 |
| NL OMVs | 5 (5) | 5 (5) | 5/5 | ||
| NM OMVs | 5 (5) | 5 (5) | 5/5 | ||
| 2 × 107 | None | 0 | 0 | 0/5 | |
| NL OMVs | 3 (3) | 1 (1) | 1/5 | ||
| NM OMVs | 1 (1) | 1 (1) | 1/5 | ||
NL, N. lactamica; NM, N. meningitidis.
Separation of N. lactamica OMPs to identify protective components.
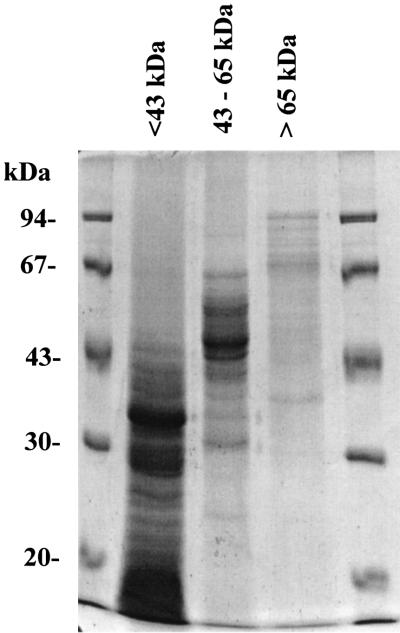
As a first step in the identification of the antigens responsible for protection against meningococcal challenge, outer membrane proteins (OMPs) from a detergent extract of N. lactamica Y92-1009 were separated by nondenaturing preparative electrophoresis. Separated proteins were pooled into three groups according to approximate molecular mass, <43 kDa, 43 to 67 kDa, and >67 kDa (Fig. 1).
FIG. 1.
SDS-PAGE of pools of preparative electrophoresis fractions. Fractions were pooled to include proteins of approximately <43 kDa, 43 to 65 kDa, and >65 kDa. Molecular size markers are in the outer lanes. Some proteins can be seen beyond these molecular sizes under the denaturing conditions used.
Groups of five mice were each immunized with equal amounts of protein (10 μg per dose) from one of these pools or with the detergent extract; they were then challenged on day 35 with N. meningitidis K454. Immunization with the <43-kDa protein pool provided the most protection against both challenge doses used (Table 5); indeed the only survivors at the high challenge dose (5 × 108 CFU) were from this group. N. lactamica proteins with a molecular mass of >67 kDa offered poor protection, with only one of five mice surviving beyond 24 h postchallenge with 2 × 107 CFU of N. meningitidis and none in the 5 × 108 CFU challenge group.
TABLE 5.
Immunization of mice with pools of proteins separated by preparative electrophoresis and protection against intraperitoneal challenge with N. meningitidis K454a
| Challenge dose (CFU) | Vaccineb | No. of survivors (no. healthy)
|
No. of survivors/ no. in group at 72 h | |
|---|---|---|---|---|
| 24 h | 52 h | |||
| 2 × 107 | None | 5 (0) | 0 | 0/5 |
| DE of whole cells | 5 (0) | 4 (2) | 4/5 | |
| <43-kDa pool | 5 (0) | 5 (5) | 5/5 | |
| 43-65-kDa pool | 5 (0) | 4 (0) | 4/5 | |
| >67-kDa pool | 5 (0) | 1 (0) | 1/5 | |
| 5 × 108 | No vaccine | 5 (0) | 0 | 0/5 |
| DE of whole cells | 5 (0) | 1 (0) | 0/5 | |
| <43-kDa pool | 5 (0) | 3 (0) | 3/5 | |
| 43-65-kDa pool | 5 (0) | 0 | 0/5 | |
| >67-kDa pool | 5 (0) | 0 | 0/5 | |
Vaccines were prepared in Freund's complete adjuvant (first immunization) or Freund's incomplete adjuvant (subsequent immunizations).0 activity plus two standard deviations (83 ELISA units).
DE, detergent (Elugent) extract of N. lactamica whole cells.
The protection observed for the <43-kDa protein pool was then compared with that with N. lactamica OMVs in larger groups of mice (n = 20) with Alhydrogel as the adjuvant (Table 6), because, unlike Freund's adjuvant, it is acceptable for use in human vaccines. Analysis of the survivors at 26 h postinfection (the time of the last death in the unvaccinated group) revealed that both the N. lactamica OMV vaccine and the <43-kDa protein pool invoked significant protection compared with the unvaccinated group (P < 0.001; chi-square test with Yates' correction). However, there were fewer survivors in the group vaccinated with the <43-kDa protein pool compared with the N. lactamica OMV vaccine (P < 0.05). In day 35 sera from these mice, a bactericidal titer of <4 was observed against meningococcal strains Z3524, NZ394/98, BZ198, BZ10, K454, FAM18, and C11 (data not shown).
TABLE 6.
Immunization of mice with N. lactamica OMVs or <43-kDa protein pool protects against intraperitoneal challenge with N. meningitidis K454a
| Challenge dose (CFU) of N. meningitidis K454 | Vaccine | No. of survivors (no. healthy)
|
No. of survivors/ no. in group at 72 h | |
|---|---|---|---|---|
| 24 h | 52 h | |||
| 4.4 × 106 | None | 0 | 0 | 0/20 |
| OMVs | 20 (20) | 20 (20) | 20/20 | |
| <43-kDa pool | 19 (11) | 14 (14) | 14/20 | |
| 8.7 × 107 | None | 1 (0) | 0 (0) | 0/20 |
| OMVs | 15 (1) | 5 (3) | 3/20 | |
| <43-kDa pool | 8 (1) | 2 (2) | 2/20 | |
Both the OMVs and <43-kDa protein pool were prepared with Alhydrogel as the adjuvant. At the lower dose at 24 h postinfection (the time of the last death in the unvaccinated group), protection was significant for both vaccines compared with the unvaccinated group (P <0.001, chi-square test with Yates' correction); in addition, there was a significant difference between the number of survivors in the group vaccinated with the <43-kDa protein pool compared with the OMV vaccine (P < 0.05).
Protection by <43-kDa pool and N. lactamica OMVs against experimental infection with different isolates of N. meningitidis.
Protection provided by the <43-kDa pool and N. lactamica OMVs against challenge with meningococcal isolates B16B6, MC58 (serogroup B), L91-543, and GN (serogroup C) was determined with the mouse intraperitoneal challenge model (Table 7). More survivors were observed in the vaccinated groups than in the nonvaccinated controls, demonstrating that the N. lactamica <43-kDa protein pool and OMV vaccines protected against challenge by each of these strains. The variation in numbers of survivors, particularly at the high challenge dose, reflects inherent differences in virulence between the meningococcal strains used in the mouse model.
TABLE 7.
Protection of mice against serogroup B and C meningococcal challenge following immunization with N. lactamica OMVs or <43-kDa protein pool and Alhydrogel as the adjuvant
| N. meningitidis challenge strain | Challenge dose (CFU) | Vaccine | No. of survivors (no. healthy)
|
No. of survivors/ no. in group at 72 h | |
|---|---|---|---|---|---|
| 24 h | 52 h | ||||
| B16B6 | 1.3 × 106 | None | 1 (0) | 1 (1) | 1/5 |
| OMVs | 5 (5) | 5 (5) | 5/5 | ||
| <43-kDa pool | 5 (5) | 5 (5) | 5/5 | ||
| 1.6 × 108 | None | 0 | 0 | 0/5 | |
| OMVs | 3 (0) | 2 (2) | 2/5 | ||
| <43-kDa pool | 2 (0) | 2 (2) | 2/5 | ||
| MC58 | 1.0 × 107 | None | 1 (0) | 1 (1) | 1/5 |
| OMVs | 4 (2) | 4 (4) | 4/5 | ||
| <43-kDa pool | 5 (4) | 5 (5) | 5/5 | ||
| 1.5 × 108 | None | 0 | 0 | 0/5 | |
| OMVs | 2 (1) | 2 (2) | 2/5 | ||
| <43-kDa pool | 0 | 0 | 0/5 | ||
| L91-543 | 5.1 × 106 | None | 0 | 0 | 0/5 |
| OMVs | 4 (2) | 4 (4) | 4/5 | ||
| <43-kDa pool | 5 (2) | 5 (5) | 5/5 | ||
| 2.4 × 108 | None | 0 | 0 | 0/5 | |
| OMVs | 3 (0) | 3 (3) | 3/5 | ||
| <43-kDa pool | 5 (2) | 5 (5) | 5/5 | ||
| GN | 4.0 × 106 | None | 1 (0) | 1 (1) | 1/5 |
| OMVs | 5 (5) | 5 (5) | 5/5 | ||
| <43-kDa pool | 5 (5) | 5 (5) | 5/5 | ||
| 1.0 × 108 | None | 1 (1) | 1 (1) | 1/5 | |
| OMVs | 5 (5) | 5 (5) | 5/5 | ||
| <43-kDa pool | 5 (2) | 4 (4) | 4/5 | ||
DISCUSSION
In this study, we have demonstrated, for the first time, that vaccines comprised of N. lactamica antigens protect against meningococcal disease in a murine infection model.
Carriage of commensal Neisseria spp., particularly N. lactamica, has been considered important for the development and maintenance of natural immunity to meningococcal disease in young children, and both antibody cross-reactivity and bactericidal antibodies to meningococci have been determined following N. lactamica carriage in humans (10). Cross-reacting bactericidal antibodies between meningococci and N. lactamica were demonstrated in a survey of schoolchildren following an outbreak of meningococcal disease (30), in which bactericidal activity of sera toward the outbreak strain could be reduced by adsorption of the sera with N. lactamica. In contrast, a study with sera obtained from patients indicated a lack of association between antibodies to meningococcal OMPs and carriage of N. lactamica (18).
In the present study, antibodies raised against OMVs prepared from N. lactamica and N. meningitidis showed a high degree of cross-reactivity with a range of meningococcal strains by whole-cell ELISA. Bactericidal antibodies were not observed in this study in sera from mice immunized with N. lactamica vaccines, even though mice immunized with the same preparations were protected against meningococcal challenge. It is becoming apparent that bactericidal antibody titer may not be a good correlate of protection in the mouse meningococcal infection model. Bactericidal antibodies are undoubtedly a good correlate with protection for meningococcal vaccines based on capsular polysaccharide (3, 11) and are important for prediction of protection provided by meningococcal OMV vaccines containing PorA (8), which do elicit bactericidal antibodies in mice.
The lack of in vitro bactericidal activity in sera from mice immunized with N. lactamica indicates that other protective mechanisms, such as opsonophagocytosis, may be operating. Protection in mice in the absence of bactericidal antibodies has also been observed following immunization with meningococcal transferrin binding protein A (TbpA) (28). Human antibodies against TbpA and -B following disease have been demonstrated to have opsonic activity (19). It will therefore be important to assess human immune responses to N. lactamica-based vaccines and determine the range of functional responses elicited.
This study has confirmed the vaccine potential of N. lactamica with a mouse intraperitoneal challenge model. We recognize that this is not an ideal model of meningococcal disease, as it does not follow the natural pathogenesis of the disease in humans and an exogenous iron source is required to establish meningococcemia. However, this model of bacteremia allows assessment of active immunization and protection. It also permits comparison of the protection provided by different vaccines (12), and a range of challenge strains can be used. With this model, we were able to confirm that N. lactamica whole cells and various antigen preparations protected against challenge by meningococcal isolates representing different clonal lineages belonging to serogroups B and C.
The <43-kDa protein pool provided protection greater than or equivalent to that afforded by the original detergent extract of whole cells, indicating that protective components may have been concentrated in this fraction. Cross-reaction between the LOS of N. meningitidis and N. lactamica has been demonstrated previously (17). However, the role of LOS in protection in this study is not clear. Purified N. lactamica LOS did not protect against meningococcal challenge, but the experimental N. lactamica vaccines described here contain LOS in various amounts, perhaps in a more native and immunogenic form. Experiments are in progress to determine the relative contribution of protein and LOS components to protection.
Existing meningococcal vaccines based on capsular polysaccharide offer protection that is serogroup specific, and the effectiveness of vaccines based on meningococcal OMVs is affected by variation in the immunodominant serosubtyping antigen PorA (21). Unlike N. meningitidis, N. lactamica does not possess PorA (17), potentially enabling it to provide protection that is not serosubtype specific. The protection observed against a diverse panel of meningococcal isolates is further evidence that N. lactamica has the potential to provide a serogroup- and serosubtype-independent vaccine against meningococcal disease. Such a vaccine could be based on isolated antigens, OMVs, or live organisms. There has been discussion of construction of live attenuated meningococcal vaccines for intranasal use (25). This study suggests that N. lactamica would be a better, and potentially safer, alternative to this approach, mimicking and enhancing the natural immunization that occurs through natural carriage.
Acknowledgments
This work was funded by the Meningitis Trust and the United Kingdom Department of Health.
The views expressed in this publication are those of the authors and are not necessarily those of the Department of Health.
We particularly thank Mark Achtman for providing the panel of meningococcal isolates used in this study. MLST typing of a number of the meningococcal strains was performed by Julia Bennett, University of Oxford, Oxford, United Kingdom.
Editor: E. I. Tuomanen
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1988. Typing of group B Neisseria meningitidis with monoclonal antibodies in the whole-cell ELISA. J. Med. Microbiol. 26:177-180. [PubMed] [Google Scholar]
- 2.Ala'Aldeen, D. A., R. A. Wall, and S. P. Borriello. 1990. Immunogenicity and cross-reactivity of the 70 kDa iron-regulated protein of Neisseria meningitidis in man and animals. J. Med. Microbiol. 32:275-281. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: a reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cann, K. J., and T. R. Rogers. 1989. Detection of antibodies to common antigens of pathogenic and commensal Neisseria species. J. Med. Microbiol. 30:23-31. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright, K. A., J. M. Stuart, and N. D. Noah. 1986. An outbreak of meningococcal disease in Gloucestershire. Lancet ii:558-561. [DOI] [PubMed]
- 6.Cartwright, K. A. V., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coen, P. G., K. Cartwright, and J. Stuart. 2000. Mathematical modelling of infection and disease due to Neisseria meningitidis and Neisseria lactamica. Int. J. Epidemiol. 29:180-188. [DOI] [PubMed] [Google Scholar]
- 8.de Kleijn, E. D., R. de Groot, J. Labadie, A. B. Lafeber, G. van den Dobbelsteen, L. van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rumke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer membrane vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 18:1456-1466. [DOI] [PubMed] [Google Scholar]
- 9.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-509. [DOI] [PubMed] [Google Scholar]
- 10.Gold, R., I. Goldschneider, M. L. Lepow, T. F. Draper, and M. Randolph. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137:112-121. [DOI] [PubMed] [Google Scholar]
- 11.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorringe, A. R., K. M. Reddin, P. Voet, and J. T. Poolman. 2001. Animal models for meningococcal disease. Methods in molecular medicine, vol. 66: meningococcal vaccines: methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 13.Griffiss, J. M., B. L. Brandt, and G. A. Jarvis. 1987. Natural immunity to the meningococcus, p. 99-119. In N. A. Vedros (ed.), Evolution of meningococcal disease, vol. 2. CRC Press, Boca Raton, Fla.
- 14.Gu, X. X., and C. M. Tsai. 1991. Purification of rough-type lipopolysaccharides of Neisseria meningitidis from cells and outer vesicles in spent media. Anal. Biochem. 196:311-318. [DOI] [PubMed] [Google Scholar]
- 15.Jones, D. W., and R. H. Mallard. 1993. Age incidence of meningococcal infection, England and Wales 1984-1991. J. Infect. 27:83-88. [DOI] [PubMed] [Google Scholar]
- 16.Kaczmarski, E. B. 1997. Meningococcal disease in England and Wales: 1995. Commun. Dis. Rep. 7:R55-R59. [PubMed] [Google Scholar]
- 17.Kim, J. J., R. E. Mandrell, and J. M. Griffiss. 1989. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect. Immun. 57:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremastinou, J., G. Tzanakaki, A. Pagalis, M. Theodondou, D. M. Weir, and C. C. Blackwell. 1999. Detection of IgG and IgM to meningococcal outer membrane proteins in relation to carriage of Neisseria meningitidis or Neisseria lactamica. FEMS Immunol. Med. Microbiol. 24:73-78. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann, A. K., A. R. Gorringe, K. M. Reddin, K. West, I. Smith, and A. Halstensen. 1999. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect. Immun. 67:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden, M. C., and B. G. Spratt. 1999. Meningococcal conjugate vaccines: new opportunities and new challenges. Lancet 354:615-616. [DOI] [PubMed] [Google Scholar]
- 21.Martin, S. L., R. Borrow, P. van der Ley, M. Dawson, A. J. Fox, and K. A. V. Cartwright. 2000. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine 18:2476-2481. [DOI] [PubMed] [Google Scholar]
- 22.Miller, E., D. Salisbury, and M. Ramsay. 2002. Planning, registration and implementation of an immunization campaign against meningococcal serogroup C disease in the United Kingdom: a success story. Vaccine 20:S58-S67. [DOI] [PubMed] [Google Scholar]
- 23.Olsen, S. F., B. Djurhuus, K. Rasmussen, D. H. Joensen, S. O. Larsen, H. Zoffman, and I. Lind. 1991. Pharyngeal carriage of Neisseria meningitidis and Neisseria lactamica in households with infants within areas with high and low incidences of meningococcal disease. Epidemiol. Infect. 106:445-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsay, M. E., A. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 25.Tang, C., R. Moxon, and M. M. Levine. 1999. For discussion: live attenuated vaccines for group B meningococcal disease. Vaccine 17:114-117. [DOI] [PubMed] [Google Scholar]
- 26.Troncoso, G., S. Sanchez, M. Moreda, M. T. Criado, and C. M. Ferreiros. 2000. Antigenic cross-reactivity between outer membrane proteins of Neisseria meningitidis and commensal Neisseria species. FEMS Immunol. Med. Microbiol. 27:103-109. [DOI] [PubMed] [Google Scholar]
- 27.Vogel, U., H. Claus, and M. Frosch. 2000. Rapid serogroup switching in Neisseria meningitidis. N. Engl. J. Med. 342:219-220. [DOI] [PubMed] [Google Scholar]
- 28.West, D., K. M. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. R. Gorringe. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against meningococcal infection. Infect. Immun. 69:1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson, H. D., and T. L. Overman. 1976. Septicemia due to Neisseria lactamica. J. Clin. Microbiol. 4:214-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zorgani, A. A., V. S. James, J. Stewart, C. C. Blackwell, R. A. Elton, and D. M. Weir. 1996. Serum bactericidal activity in a secondary school population following an outbreak of meningococcal disease: effects of carriage and secretor status. FEMS Immunol. Med. Microbiol. 14:73-81. [DOI] [PubMed] [Google Scholar]