Abstract
1. Segments of mouse pancreatic or exorbital lacrimal gland were superfused with saline solutions. Under visual control two micro-electrodes were inserted into neighbouring cells within the same acinus or into neighbouring acini. Cell to cell electrical coupling was assessed by injecting rectangular current pulses through one electrode and measuring the electrotonic potential change in the same cell (V1) and in the neighbouring cell (V2). Acetylcholine (ACh) was added locally to impaled acini by micro-ionophoresis from an extracellular micropipette.
2. Exposure of the tissues to a Krebs solution equilibrated with 100% CO2 caused a rapid increase in the size of electrotonic potential changes in the current injection cell and disappearance of the electrotonic potential changes in a neighbouring acinus or cell. This electrical uncoupling of previously coupled cells was rapidly reversible upon return to a solution equilibrated with 95% O2 and 5% CO2.
3. Reduction of electrical intercellular coupling was also obtained using smaller CO2 concentrations (50, 20 or 10%). In these cases the effects developed more slowly and were less dramatic. Reducing the extracellular HCO3 concentration enhanced the uncoupling effect of 10 or 20% CO2. However, weak uncoupling effects were still observed using 10 or 20% CO2 in combination with a high bicarbonate concentration maintaining a constant extracellular pH (7·4).
4. Reductions in extracellular pH (down to 5·5) achieved by varying combinations of Tris base and Tris HCl had no effect on electrical coupling. Brief periods of anoxia (100% N2) also had no effect.
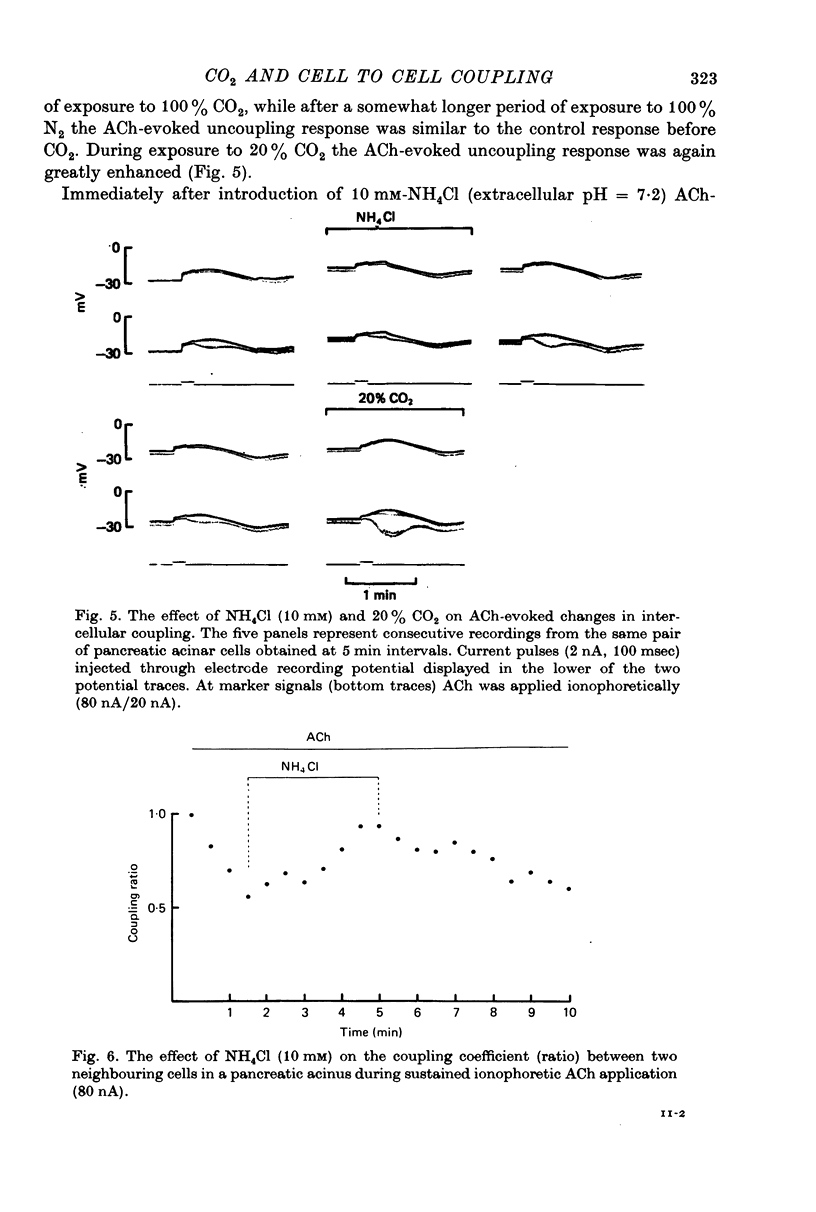
5. Exposure to 20% CO2 markedly enhanced the uncoupling effect of a brief ionophoretic pulse of ACh.
6. Exposure of the tissue to 10 mM-NH4Cl, a procedure expected to increase the intracellular pH, counteracted the uncoupling effect of ACh. During sustained uncoupling caused by a sustained ACh stimulation a brief period of exposure to NH4Cl caused an immediate and fully reversible recoupling.
7. It is concluded that variations in intracellular pH have marked effects on the electrical coupling between neighbouring cells in the pancreatic and lacrimal acinar tissue.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Honerjäger P. Influence of carbon dioxide on level of ionised calcium in squid axons. Nature. 1978 May 11;273(5658):160–161. doi: 10.1038/273160a0. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Acetylcholine-like effects of intracellular calcium application in pancreatic acinar cells. Nature. 1977 Jul 14;268(5616):147–149. doi: 10.1038/268147a0. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Electrical coupling and uncoupling of exocrine acinar cells. J Cell Biol. 1978 Nov;79(2 Pt 1):533–545. doi: 10.1083/jcb.79.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: acetylcholine-evoked electrical uncoupling and its ionic dependency. J Physiol. 1978 Jan;274:81–06. doi: 10.1113/jphysiol.1978.sp012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Increase in free Ca2+ in muscle after exposure to CO2. Nature. 1978 Sep 21;275(5677):236–238. doi: 10.1038/275236a0. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Reynafarje B., Vercesi A., Tew W. P. Transport and accumulation of calcium in mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:160–176. doi: 10.1111/j.1749-6632.1978.tb41941.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeable junctions. Cold Spring Harb Symp Quant Biol. 1976;40:49–63. doi: 10.1101/sqb.1976.040.01.008. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Rose B. Calcium in (junctional) intercellular communication and a thought on its behavior in intracellular communication. Ann N Y Acad Sci. 1978 Apr 28;307:285–307. doi: 10.1111/j.1749-6632.1978.tb41958.x. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol. 1976 Jan;254(3):583–606. doi: 10.1113/jphysiol.1976.sp011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson O. H., Iwatsuki N. The role of calcium in pancreatic acinar cell stimulus-secretion coupling: an electrophysiological approach. Ann N Y Acad Sci. 1978 Apr 28;307:599–617. doi: 10.1111/j.1749-6632.1978.tb41984.x. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L., Warner A. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature. 1977 Nov 3;270(5632):56–57. doi: 10.1038/270056a0. [DOI] [PubMed] [Google Scholar]



