Abstract
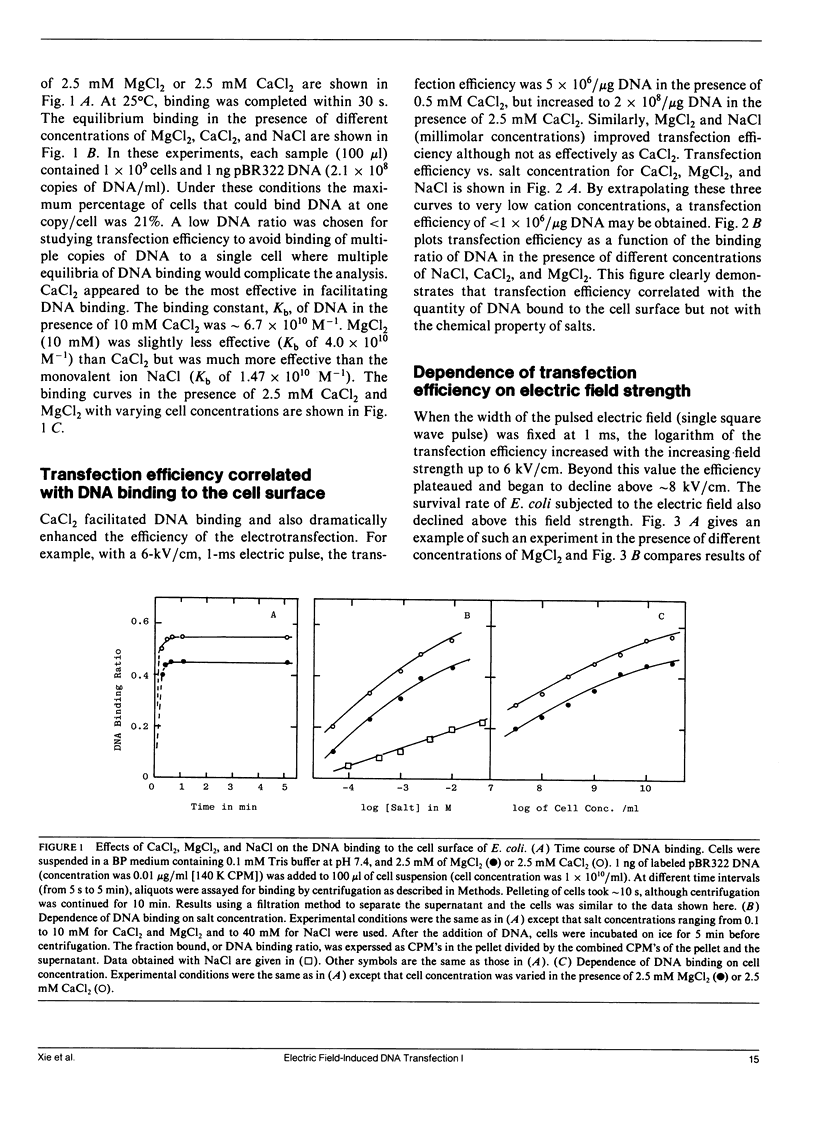
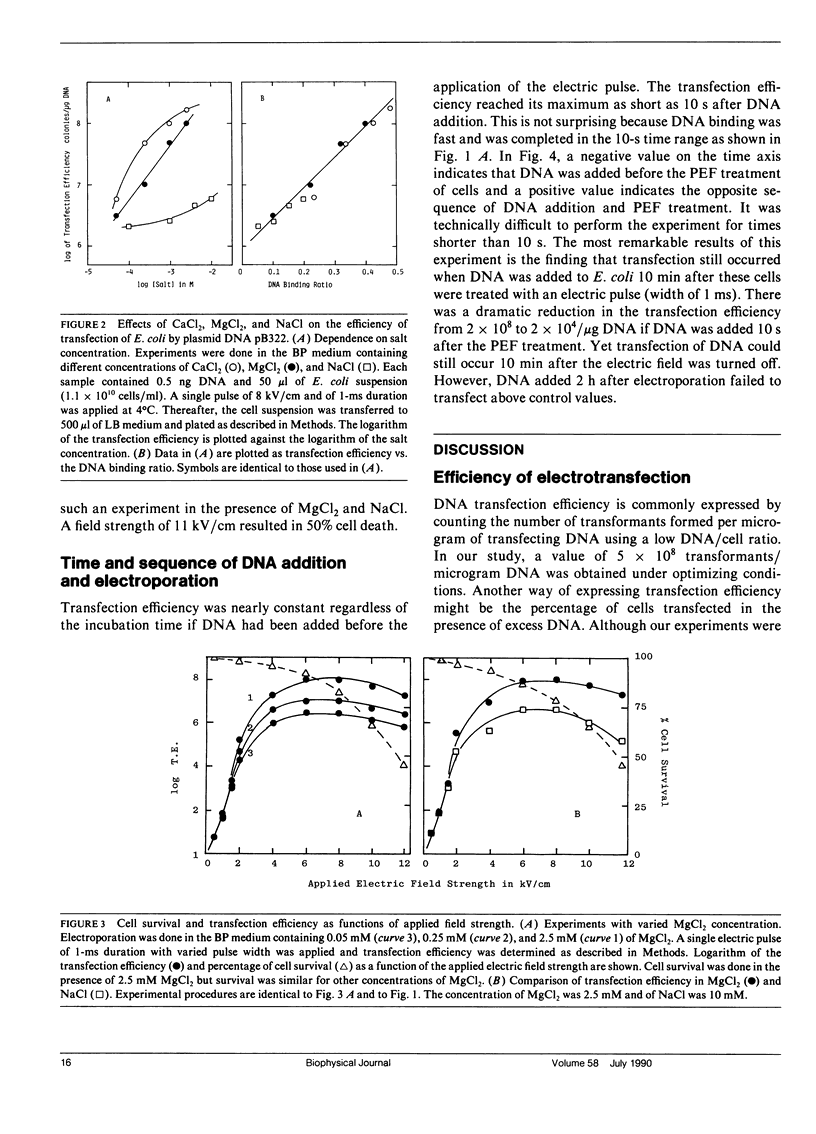
A study of mechanisms of electrotransfection using Escherichia coli (JM 105) and the plasmid DNA pBR322 as model system is reported. pBR322 DNA carries an ampicillin resistance gene: E. coli transformants are conveniently assayed by counting colonies in a selection medium containing 50 micrograms/ml ampicillin and 25 micrograms/ml streptomycin. Samples not exposed to the electric field showed no transfection. In the absence of added cations, the plasmid DNA remains in solution and the efficiency of the transfection was 2 x 10(6)/micrograms DNA for cells treated with a 8-kV/cm, 1-ms electric pulse (square wave). DNA binding to the cell membrane greatly enhanced the efficiency of the transfection and this binding was increased by milimolar concentrations of CaCl2, MgCl2, or NaCl (CaCl2 greater than MgCl2 greater than NaCl). For example, in the presence of 2.5 mM CaCl2, 55% of the DNA added bound to E. coli and the transfection efficiency was elevated by two orders of magnitude (2 x 10(8)/micrograms DNA). These ions did not cause cell aggregation. With a low ratio of DNA to cells (less than 1 copy/cell), transfection efficiency correlated with the amount of DNA bound to the cell surface irrespective of salts. When the DNA binding ratio approached zero, the transfection efficiency was reduced by two to three orders, indicating that DNA entry by diffusion through the bulk solution was less than 1%. Square pulses of up to 12 kV/cm and 1 ms were used in the electrotransfection experiments. When cell concentration was 1 x 1010 cell/ml and DNA was added before the pulse, a transfection efficiency of up to 5 x 108/ microg DNA was obtained under optimum conditions (a single pulse of 8 kV/cm, 1 ms, in the presence of 5 mM CaCl2). When DNA was added to E. coli after the electric pulse, the efficiency of the transfection was dramatically reduced owing to the resealing of pores. Transfection was reduced to zero when DNA was added 2 h after the electroporation. However, transfection as high as 5 x 104/microg DNA was still recorded when DNA was added 10 min after the electric field was turned off. Because DNA entry took place in the absence of an electric field it could not be driven by the electrophoretic forces. DNA entry was facilitated by surface binding followed by lateral diffusion of the bound DNA into the cells through the field-induced membrane pores.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Beckers F., Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979 Jul 16;48(2):181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- Calvin N. M., Hanawalt P. C. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988 Jun;170(6):2796–2801. doi: 10.1128/jb.170.6.2796-2801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. C. Cell poration and cell fusion using an oscillating electric field. Biophys J. 1989 Oct;56(4):641–652. doi: 10.1016/S0006-3495(89)82711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cymbalyuk E. S., Chernomordik L. V., Broude N. E., Chizmadzhev YuA Electro-stimulated transformation of E. coli cells pretreated by EDTA solution. FEBS Lett. 1988 Jul 4;234(1):203–207. doi: 10.1016/0014-5793(88)81334-6. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler S., Wirth R. Transformation of bacteria with plasmid DNA by electroporation. Anal Biochem. 1988 Apr;170(1):38–44. doi: 10.1016/0003-2697(88)90086-3. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. T. Hemolysis of human erythrocytes by transient electric field. Proc Natl Acad Sci U S A. 1977 May;74(5):1923–1927. doi: 10.1073/pnas.74.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Survival of sucrose-loaded erythrocytes in the circulation. Nature. 1978 Mar 16;272(5650):258–260. doi: 10.1038/272258a0. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Voltage-induced conductance in human erythrocyte membranes. Biochim Biophys Acta. 1979 Jul 5;554(2):479–497. doi: 10.1016/0005-2736(79)90386-9. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim Biophys Acta. 1977 Dec 1;471(2):227–242. doi: 10.1016/0005-2736(77)90252-8. [DOI] [PubMed] [Google Scholar]
- Neumann E., Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol. 1972 Dec 29;10(3):279–290. doi: 10.1007/BF01867861. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. T., Morgenthaler A. W., Weaver J. C. Tissue electroporation. Observation of reversible electrical breakdown in viable frog skin. Biophys J. 1989 Dec;56(6):1163–1171. doi: 10.1016/S0006-3495(89)82763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A. J., Hamilton W. A. Effects of high electric fields on micro-organisms. 3. Lysis of erythrocytes and protoplasts. Biochim Biophys Acta. 1968 Aug;163(1):37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- Serpersu E. H., Kinosita K., Jr, Tsong T. Y. Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim Biophys Acta. 1985 Feb 14;812(3):779–785. doi: 10.1016/0005-2736(85)90272-x. [DOI] [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Taketo A. DNA transfection of Escherichia coli by electroporation. Biochim Biophys Acta. 1988 Mar 31;949(3):318–324. doi: 10.1016/0167-4781(88)90158-3. [DOI] [PubMed] [Google Scholar]
- Teissie J., Tsong T. Y. Electric field induced transient pores in phospholipid bilayer vesicles. Biochemistry. 1981 Mar 17;20(6):1548–1554. doi: 10.1021/bi00509a022. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electric modification of membrane permeability for drug loading into living cells. Methods Enzymol. 1987;149:248–259. doi: 10.1016/0076-6879(87)49063-0. [DOI] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]


