Abstract
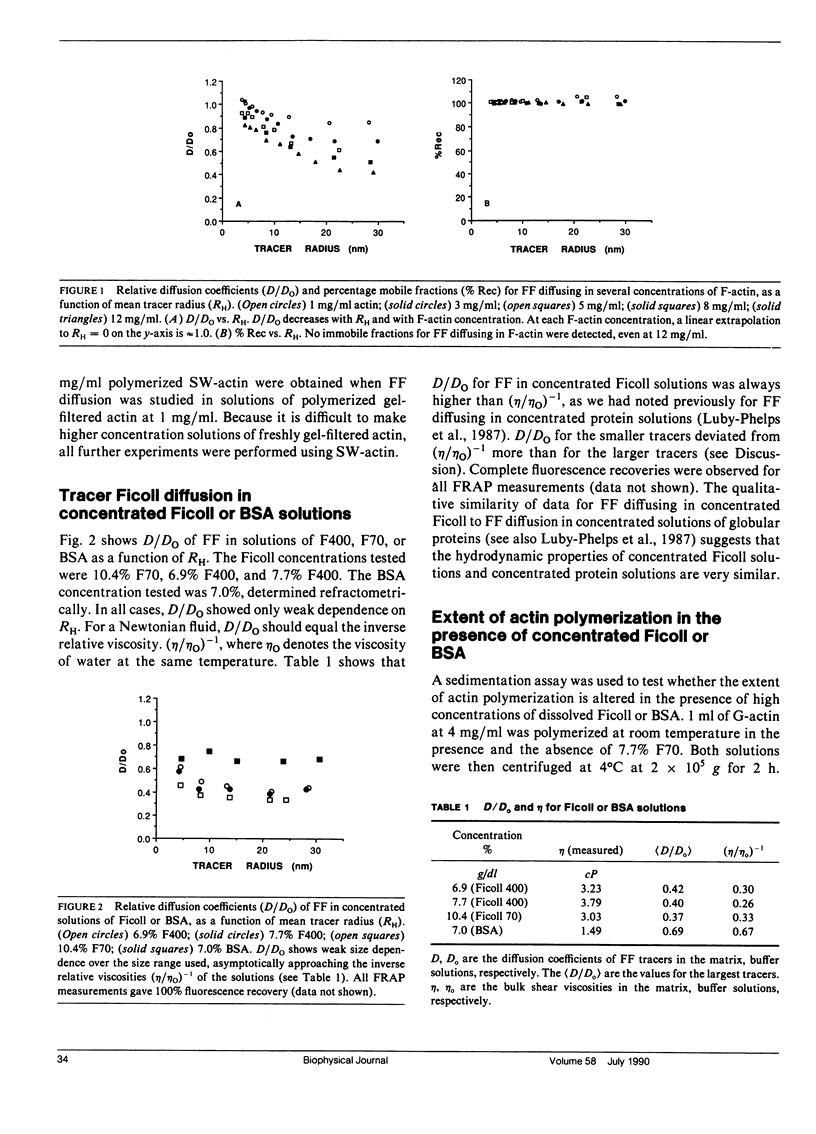
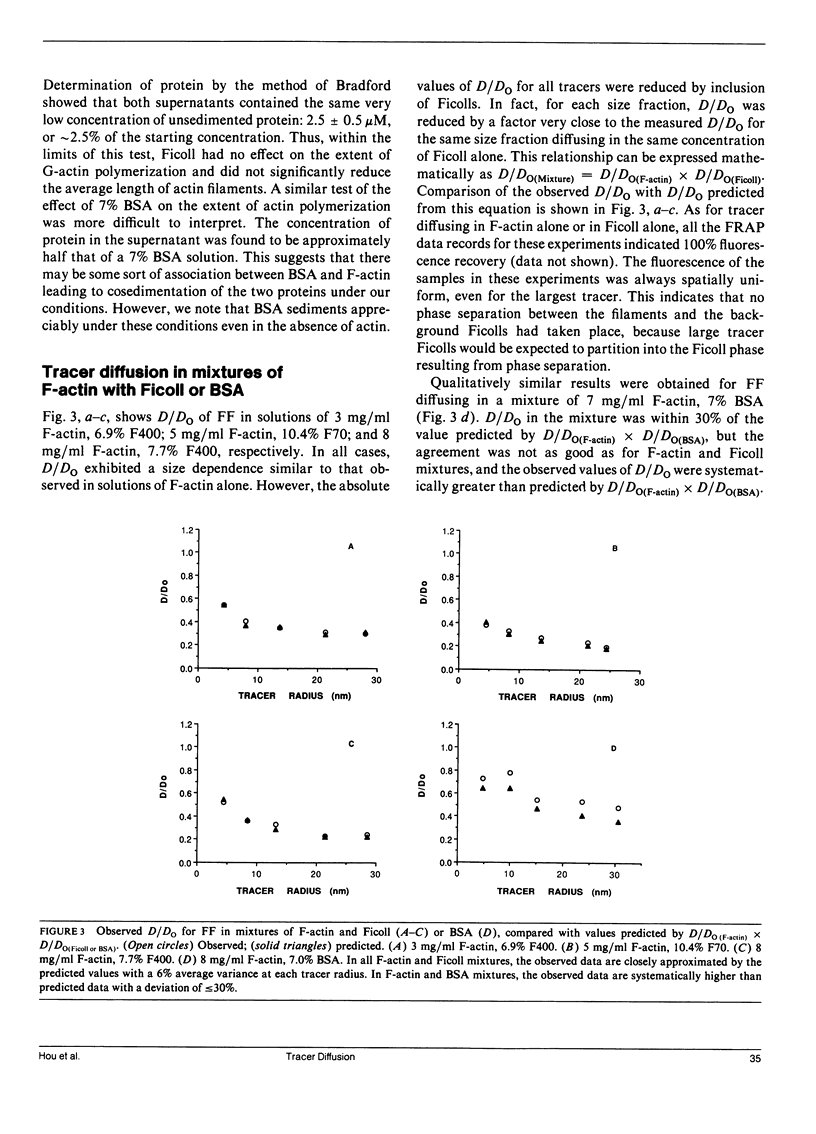
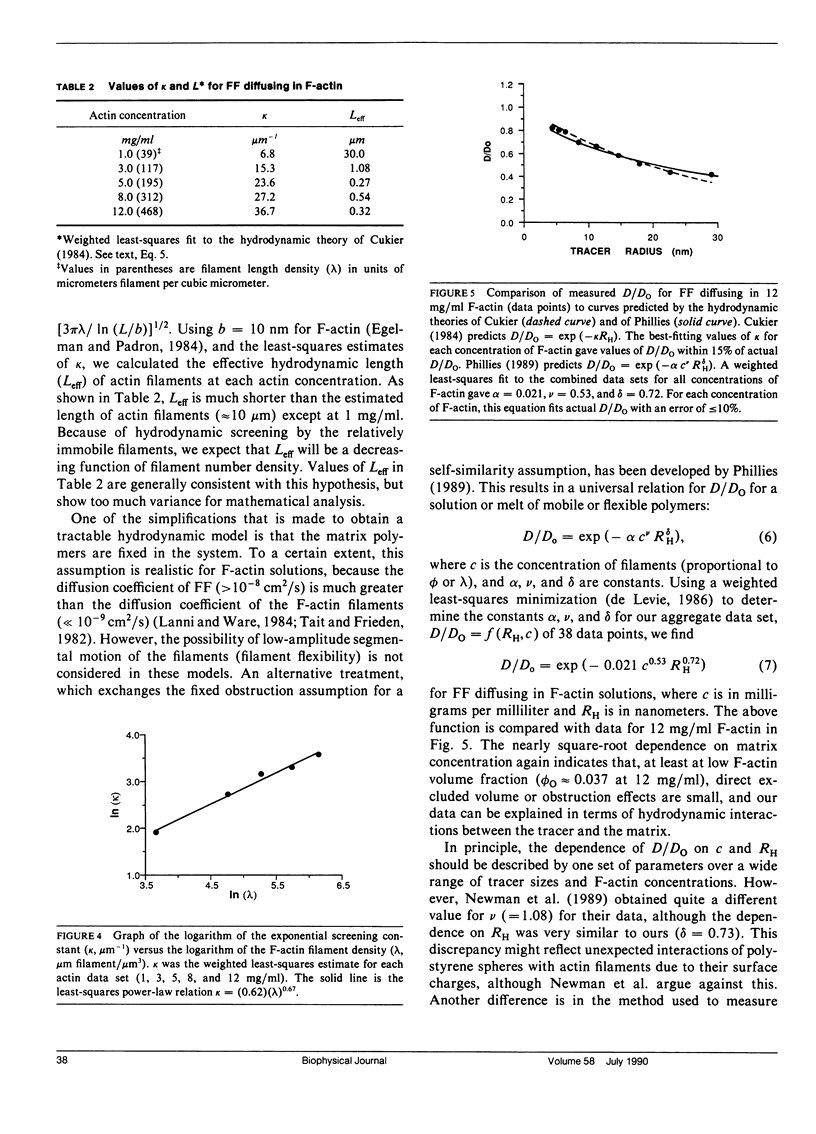
We have previously reported that self-diffusion of inert tracer particles in the cytoplasm of living Swiss 3T3 cells is hindered in a size-dependent manner (Luby-Phelps, K., D.L. Taylor, and F. Lanni. 1986. J. Cell Biol. 102:2015-2022; Luby-Phelps, K., P.E. Castle, D.L. Taylor, and F. Lanni. 1987. Proc Natl. Acad. Sci. USA. 84:4910-4913). Lacking a theory that completely explains our data, we are attempting to understand the molecular architecture responsible for this phenomenon by studying tracer diffusion in simple, reconstituted model systems. This report contains our findings on tracer diffusion in concentrated solutions of Ficoll 70 or Ficoll 400, in solutions of entangled F-actin filaments, and in solutions of entangled F-actin containing a background of concentrated Ficoll particles or concentrated bovine serum albumin (BSA). A series of size-fractionated fluorescein-Ficolls were used as tracer particles. By fluorescence recovery after photobleaching (FRAP), we obtained the mean diffusion coefficients in a dilute, aqueous reference phase (Do), the mean diffusion coefficients in the model matrices (D), and the mean hydrodynamic radii (RH) for selected tracer fractions. For each model matrix, the results were compared with similar data obtained from living cells. As in concentrated solutions of globular proteins (Luby-Phelps et al., 1987), D/Do was not significantly size-dependent in concentrated solutions of Ficoll 400 or Ficoll 70. In contrast, D/Do decreased monotonically with increasing RH in solutions of F-actin ranging in concentration from 1 to 12 mg/ml. This size dependence was most pronounced at higher F-actin concentrations. However, the shape of the curve and the extrapolated value of D/Do in the limit, RH----O did not closely resemble the cellular data for tracers in the same size range (3 less than RH less than 30 nm). In mixtures of F-actin and Ficoll or F-actin and BSA, D/Do was well approximated by D/Do for the same concentration of F-actin alone multiplied by D/Do for the same concentrations of Ficoll or BSA alone. Based on these results, it is possible to model the submicroscopic architecture of cytoplasm in living cells as a densely entangled filament network (perhaps made up of F-actin and other filamentous structures) interpenetrated by a fluid phase crowded with globular macromolecules, which in cytoplasm would be primarily proteins.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Bray D., Thomas C. The actin content of fibroblasts. Biochem J. 1975 May;147(2):221–228. doi: 10.1042/bj1470221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella J. F., Maack D. J. Cap Z(36/32) is a contaminant and the major inhibitor of actin network formation in conventional actin preparations. Biochem Biophys Res Commun. 1987 May 29;145(1):625–630. doi: 10.1016/0006-291x(87)91366-0. [DOI] [PubMed] [Google Scholar]
- Chandler D., Weeks J. D., Andersen H. C. Van der waals picture of liquids, solids, and phase transformations. Science. 1983 May 20;220(4599):787–794. doi: 10.1126/science.220.4599.787. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Qian H. Interpretation of fluorescence correlation spectroscopy and photobleaching recovery in terms of molecular interactions. Methods Cell Biol. 1989;30:307–332. doi: 10.1016/s0091-679x(08)60984-x. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Gershon N. D., Porter K. R., Trus B. L. The cytoplasmic matrix: its volume and surface area and the diffusion of molecules through it. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5030–5034. doi: 10.1073/pnas.82.15.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. J Mol Biol. 1979 Nov 5;134(3):539–553. doi: 10.1016/0022-2836(79)90366-8. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K. Radioimmunoassay for tubulin: a quantitative comparison of the tubulin content of different established tissue culture cells and tissues. Cell. 1978 Aug;14(4):795–804. doi: 10.1016/0092-8674(78)90335-5. [DOI] [PubMed] [Google Scholar]
- Houk T. W., Jr, Ue K. The measurement of actin concentration in solution: a comparison of methods. Anal Biochem. 1974 Nov;62(1):66–74. doi: 10.1016/0003-2697(74)90367-4. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Peetermans J., Zaner K. S., Stossel T. P., Tanaka T. Structure and mobility of actin filaments as measured by quasielastic light scattering, viscometry, and electron microscopy. J Biol Chem. 1986 Jun 25;261(18):8357–8362. [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Electron microscopic particle length of F-actin polymerized in vitro. J Biochem. 1970 Mar;67(3):437–457. doi: 10.1093/oxfordjournals.jbchem.a129267. [DOI] [PubMed] [Google Scholar]
- LAUFFER M. A. Theory of diffusion in gels. Biophys J. 1961 Jan;1:205–213. doi: 10.1016/s0006-3495(61)86884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni F., Waggoner A. S., Taylor D. L. Structural organization of interphase 3T3 fibroblasts studied by total internal reflection fluorescence microscopy. J Cell Biol. 1985 Apr;100(4):1091–1102. doi: 10.1083/jcb.100.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni F., Ware B. R. Detection and characterization of actin monomers, oligomers, and filaments in solution by measurement of fluorescence photobleaching recovery. Biophys J. 1984 Jul;46(1):97–110. doi: 10.1016/S0006-3495(84)84002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C. Determination of the structure of agarose gels by gel chromatography. Biochim Biophys Acta. 1967 Mar 22;136(2):199–205. doi: 10.1016/0304-4165(67)90064-5. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Granath K. A. Fractionation of dextran and Ficoll by chromatography on Sephadex G-200. Biochim Biophys Acta. 1967 Mar 22;136(2):191–198. doi: 10.1016/0304-4165(67)90063-3. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Castle P. E., Taylor D. L., Lanni F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4910–4913. doi: 10.1073/pnas.84.14.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Lanni F., Taylor D. L. The submicroscopic properties of cytoplasm as a determinant of cellular function. Annu Rev Biophys Biophys Chem. 1988;17:369–396. doi: 10.1146/annurev.bb.17.060188.002101. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K. Preparation of fluorescently labeled dextrans and ficolls. Methods Cell Biol. 1989;29:59–73. doi: 10.1016/s0091-679x(08)60187-9. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L. Subcellular compartmentalization by local differentiation of cytoplasmic structure. Cell Motil Cytoskeleton. 1988;10(1-2):28–37. doi: 10.1002/cm.970100107. [DOI] [PubMed] [Google Scholar]
- Nagle B. W., Doenges K. H., Bryan J. Assembly of tubulin from cultured cells and comparison with the neurotubulin model. Cell. 1977 Nov;12(3):573–586. doi: 10.1016/0092-8674(77)90258-6. [DOI] [PubMed] [Google Scholar]
- Newman J., Mroczka N., Schick K. L. Dynamic light scattering measurements of the diffusion of probes in filamentous actin solutions. Biopolymers. 1989 Feb;28(2):655–666. doi: 10.1002/bip.360280209. [DOI] [PubMed] [Google Scholar]
- Oosawa F. Size distribution of protein polymers. J Theor Biol. 1970 Apr;27(1):69–86. doi: 10.1016/0022-5193(70)90129-3. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R., Furukawa R. H., Ware B. R., Taylor D. L. The molecular mobility of alpha-actinin and actin in a reconstituted model of gelation. Cell Motil Cytoskeleton. 1988;11(1):64–82. doi: 10.1002/cm.970110107. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Gough A., Urbanik E., Wang F., Lanni F., Ware B. R., Taylor D. L. Analysis of rhodamine and fluorescein-labeled F-actin diffusion in vitro by fluorescence photobleaching recovery. Biophys J. 1988 Nov;54(5):801–815. doi: 10.1016/S0006-3495(88)83018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Tait J. F., Frieden C. Polymerization and gelation of actin studied by fluorescence photobleaching recovery. Biochemistry. 1982 Jul 20;21(15):3666–3674. doi: 10.1021/bi00258a022. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S. Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol. 1979;56:57–144. doi: 10.1016/s0074-7696(08)61821-5. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Fechheimer M. Cytoplasmic structure and contractility: the solation--contraction coupling hypothesis. Philos Trans R Soc Lond B Biol Sci. 1982 Nov 4;299(1095):185–197. doi: 10.1098/rstb.1982.0125. [DOI] [PubMed] [Google Scholar]
- Wolf D. E. Designing, building, and using a fluorescence recovery after photobleaching instrument. Methods Cell Biol. 1989;30:271–306. doi: 10.1016/s0091-679x(08)60983-8. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguerabide J., Schmidt J. A., Yguerabide E. E. Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophys J. 1982 Oct;40(1):69–75. doi: 10.1016/S0006-3495(82)84459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



