Abstract
Alveolar and cystic hydatidosis are caused by infection with the larval stages of Echinococcus multilocularis and Echinococcus granulosus, respectively. A host-protective antigen has been identified in E. granulosus. Here we identify the presence of a closely related protein in E. multilocularis, characterize and express a cDNA encoding the antigen (designated EM95), determine the structure of the em95 gene, and demonstrate that the EM95 recombinant protein can be used to induce significant levels of protection against challenge infection with E. multilocularis eggs in mice.
Echinococcus multilocularis is a cestode parasite that causes the often-fatal disease alveolar echinococcosis in humans (23). The normal life cycle of the parasite occurs between foxes, dogs or cats (the definitive hosts), and various species of rodent that act as natural intermediate hosts. Humans become infected by accidental ingestion of the parasite's eggs from the feces of an infected definitive host. Echinococcus granulosus has a similar prey-predator life cycle, with dogs as definitive hosts and herbivorous animals as intermediate hosts. Infection in humans causes cystic hydatid disease, which is a major cause of human morbidity and mortality worldwide. A recombinant vaccine has been developed for use in the control of cystic hydatid disease. The recombinant antigen, designated EG95, induces up to 100% protection in sheep against challenge infection with E. granulosus (12). EG95 has been demonstrated to be a highly effective ovine vaccine in New Zealand, Australia, and Argentina (11), and the vaccine may prove to be a useful tool in the control of hydatid disease in areas of endemicity. The potential use of the EG95 vaccine for direct use in prevention of hydatid disease in human infection has also been proposed (9, 10).
Although human infections with E. multilocularis are uncommon in many countries where the disease is endemic (7, 19), the disease has high prevalence in areas such as south Gansu, China (4). One option for reducing the impact of the disease in these areas would be to develop an effective vaccine for humans. The information available about the EG95 vaccine for E. granulosus may provide the opportunity to identify a homologous protein in E. multilocularis that could possibly be used as a vaccine antigen for the latter species.
This report describes the molecular cloning and characterization of a gene expressed in E. multilocularis whose product is homologous to EG95 and demonstrates the immunogenicity of the expressed protein in the parasite's intermediate host.
Genomic DNA was extracted from E. multilocularis protoscoleces following collection from the liver cysts of a cotton rat (Sigmodon hispidus) experimentally infected with eggs obtained from a dog-cotton rat life cycle maintained at the Hokkaido Institute of Public Health. The parasite was originally isolated from a red-backed vole (Clethrionomys rufocanus bedfordiae) from Nemuro, Hokkaido, Japan. Protoscoleces were washed several times in sterile saline and fixed in 70% ethanol. Genomic DNA was extracted as described previously (14, 25).
mRNA from E. multilocularis protoscoleces, originally obtained from a naturally infected common vole, Microtus arvalis (Swabian Jura, southwestern Germany), and maintained by repeated intraperitoneal passages, was used to prepare a cDNA library in the bacteriophage λZapII XR (Stratagene) according to the manufacturer's recommendations. The cDNA was inserted into the EcoRI and XhoI sites of the vector (15). The amplified library was screened by hybridization of eg95-labeled cDNA as described in reference 25, and stringency washes were performed at 65°C in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). One positive clone was identified from approximately 700,000 recombinant phage. The clone, designated em95, was excised from λZapII XR by R408 helper phage superinfection according to the manufacturer's recommendations (Stratagene).
Genomic DNA obtained from protoscoleces of E. multilocularis (described above) was digested with EcoRI and separated by agarose gel electrophoresis. DNA fragments were recovered using Geneclean (Bio 101) and ligated to EcoRI-digested λZapII (Stratagene). Ligation and phage packaging were performed according to the manufacturer's recommendations. The genomic DNA library was screened by hybridization with a digoxigenin-labeled em95 cDNA probe followed by in vivo excision as described above.
Phagemid DNA containing the em95 cDNA or the em95 gene was prepared using Wizard DNA purification (Promega) and used as a template in DNA sequencing reactions. Sequencing was performed using the Applied Biosystems PRISM automated sequencing system with the ABI PRISM Dye Terminator Cycle Sequencing kit. DNA and protein sequence was compiled using MacVector (version 6.5; Oxford Molecular) sequence analysis software.
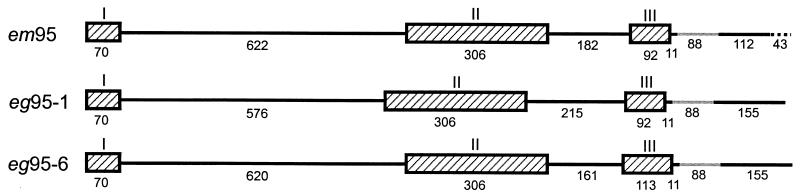
A 2,634-bp genomic DNA fragment was isolated from the E. multilocularis genomic DNA library by DNA hybridization. The em95 gene consists of three exons and two introns, with similarities in structure to the eg95 gene family of E. granulosus (Fig. 1) (3). The predicted intron splice sites of the em95 gene are conserved in comparison with the intron-exon boundaries of eg95. The lengths of exons I, II, and III (70, 306, and 92 bp, respectively) are identical to those of the eg95-1 gene (Fig. 1). Intron length of the em95 gene does not appear to be as conserved, since intron II of the E. multilocularis gene (Fig. 1) differs compared with eg95-1 and eg95-6. The em95 gene was cloned from E. multilocularis genomic DNA of Japanese origin, while the cDNA was cloned from parasite mRNA of German origin. Despite this, exons I, II, and III of the gene and cDNA are identical in DNA sequence.
FIG. 1.
Diagrammatic representation of the gene structure of em95 from E. multilocularis compared with the gene structure of E. granulosus eg95-1 and eg95-6 from Chow et al. (3). The genes of each species have a conserved structure consisting of three exons (hatched bars designated I to III) and two introns (black solid lines). Grey lines represent introns in the 3′ untranslated region, and a dotted line in the em95 gene represents a deletion (lacking consensus splice sites) in comparison with the cloned mRNA. Numbering below each exon and intron is in base pairs.
The mRNA sequence predicted from the em95 gene comprises 591 nucleotides, with an open reading frame of 468 bases. The em95 cDNA cloned from the E. multilocularis protoscolex cDNA library comprises 570 nucleotides, with an open reading frame of 447 bp. Alignment of the em95 cDNA with the gene and lack of an ATG at the 5′ end indicate that em95, like eg95, represents an incomplete copy of the mRNA and lacks the sequence encoding seven amino acids. The em95 cDNA contains two deletions in the 3′ untranslated region compared with the em95 gene (Fig. 1) (88 and 43 bp). The 88-bp deletion contains nucleotides which encode splice junctions conserved in eukaryotic genes (20). Sequence elements in the 3′ untranslated region of mRNAs have been shown to affect localization, stability, and translation of mRNAs (reviewed in reference 6), and these elements identified in the eg95 mRNA may have a similar function. eg95 transcripts lacking the 3′ untranslated region elements, like em95, have been cloned (3).
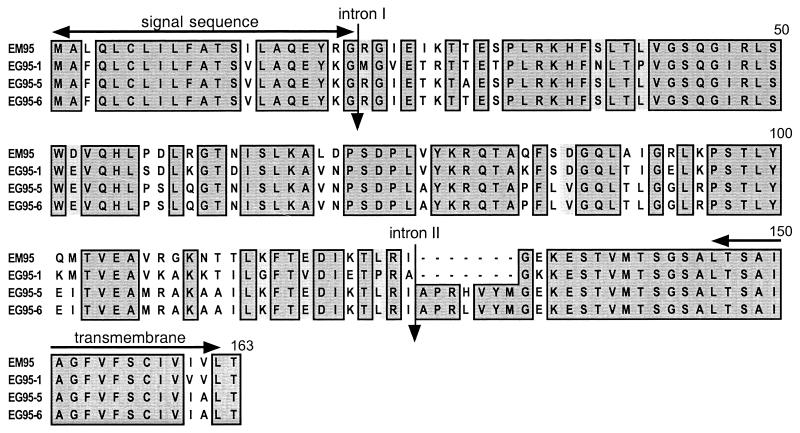
The em95 cDNA, which represents the expressed gene product of em95, was cloned from the protoscolex life cycle stage of E. multilocularis. In E. granulosus the EG95 antigen is expressed by a gene which is a member of the family comprising at least six genes which are known to be expressed (3). Only two of these genes, designated eg95-5 and eg95-6, were found to be expressed in protoscoleces, and based on this observation and the fact that the predicted amino acid sequence shows 80 and 84% identity to proteins encoded by eg95-1 and eg95-6, respectively (Fig. 2), it would appear that em95 represents the homologue of eg95-6. However, a possibly significant feature of the comparison between em95 and the eg95 gene family is that, like eg95-1, em95 lacks a 7-amino-acid component of exon 3 present in eg95-6 (Fig. 2) (3), suggesting that em95 may be more closely related to eg95-1 than it is to eg95-6.
FIG. 2.
Comparison of EM95 with proteins encoded by members of the eg95 gene family of E. granulosus. CLUSTAL W (22) alignment of the predicted amino acid sequence of EM95 with EG95 proteins was carried out. Amino acid identities are on a dark shaded background, and similarities (according to Dayhoff [5]) are on a lightly shaded background. Amino acid number is shown on the right-hand side of the sequence and represents the residue number from the initiator Met of EG95-6. Dashes indicate gaps introduced to maintain optimal alignment. Amino acids 1 to 7 of EM95 have been determined from the em95 genomic sequence. The EM95 protein used in the vaccine trials (Table 1) lacked amino acids 1 to 7.
The deduced protein encoded by em95 (Fig. 2) shows that it consists of 156 amino acids and has a predicted molecular mass of 17.1 kDa. The EM95 protein has a relatively high (13%) proportion of leucine residues and two putative N-linked glycosylation sites at amino acid positions 63 and 111 (Fig. 2). The amino acid sequence of EM95 shows 80 and 84% identity (5) to the proteins EG95-1 and EG95-6 of E. granulosus, respectively. Comparison of the predicted amino acid sequence of EM95 with EG95 (Fig. 2) indicates that 32% of the observed amino acid differences encode lysine in either EM95 or EG95-1. A highly conserved region near the carboxy terminus of the EM95 and EG95 proteins (amino acids 128 to 152 [Fig. 2]) contains the immunodominant region of EG95 identified by Woollard et al. (26). A BLAST search (1) also identified significant homology to TSA9 of Taenia saginata (13), proteins encoded by the 45W gene family of Taenia ovis (24), and other taeniid oncosphere proteins which contain fibronectin type III domains (described in reference 9). The structure predicted from the amino acid sequence of EM95 consists of one fibronectin type III domain (amino acids 34 to 119 [Fig. 2]), a hydrophobic N terminus (amino acids 1 to 16 [Fig. 2]) predicted to be a secretory signal, and a hydrophobic C terminus (amino acids 139 to 155 [Fig. 2]) encoding a transmembrane domain or glycosylphosphatidylinositol membrane anchor (8, 16, 17). These features combined suggest that the EM95 protein is extracellular and secreted.
The em95 cDNA was excised from pBluescript SK(−) using EcoRI and XhoI, subcloned into EcoRI/XhoI-digested pGEX3EX (21), and transformed into Escherichia coli BB4 strain. Following overnight growth of the bacteria, 10-fold dilution in fresh culture medium, and induction with 0.2 mM isopropyl-β-d-thiogalactopyranoside for at least 3 h, EM95 was expressed as a fusion protein with glutathione S-transferase (GST) and purified by affinity chromatography according to the method of Smith and Johnson (21).
Two vaccine trials were undertaken to determine whether the EM95 protein could induce host protective immunity against infection with E. multilocularis infection. In trial 1, 6-week-old female BALB/c mice were each immunized subcutaneously with 20 μg of EM95-GST fusion protein plus 50 μg of saponin (Sigma) or with 20 μg of GST plus 50 μg of saponin. At 2 and 4 weeks, each mouse received a second injection identical to the primary immunization. Two weeks after the final immunization all mice were orally challenged with 3,000 E. multilocularis eggs isolated from the intestines of naturally infected red foxes (Vulpes vulpes) which were necropsied within a control program in an area of high endemicity in southwest Germany (18). The gut contents of infected foxes was washed several times with phosphate-buffered saline (PBS). Worms were allowed to sediment by gravity. Mature proglottids and eggs were separated by centrifugation through a 20% Percoll solution (Sigma) in PBS at 2,500 × g for 5 min. The pellet was digested using proteinase K (5 mg/ml; Boehringer) for 30 min at 37°C. E. multilocularis eggs were separated from debris by centrifugation in 60% Percoll at 2,500 × g for 5 min and stored in PBS at 4°C until use. Egg viability and infection dose were determined in vivo by oral inoculation into common voles (M. arvalis). All procedures involving infectious material were performed in a specially designed safety laboratory (biosafety level 3). All mice were necropsied, and the number of alveolar echinococcosis cysts was counted for each mouse (Table 1). Vaccination with the control recombinant protein, GST, resulted in a reduction in the mean number of alveolar hydatid cysts which established in mice compared with the number that established in mice injected with buffer alone. Mice immunized with the EM95 recombinant protein developed significantly fewer cysts (78.5%) following the challenge infection in comparison with the GST control mice (Table 1, comparison C versus D, P < 0.05). In a second trial, vaccination and control groups of mice received a regimen of treatment similar to that used in trial 1, with the addition of a control and vaccination groups which used the oil adjuvant Squalane-Tween 80-pluronic L121 (STP) (2). Again, there was a notable decrease in the number of cysts establishing in mice vaccinated with the GST control protein in comparison with mice immunized with buffer alone or with buffer plus adjuvant alone. In this trial the effect was statistically significant (Table 1, comparison G versus I, P < 0.01). Immunization with the EM95 antigen induced statistically significant protection against infection with E. multilocularis in comparison with mice immunized with the control GST protein. This was evident when either saponin or oil was used as adjuvant. Immunization with EM95 plus saponin induced 82.9% protection (Table 1, comparison H versus J, P < 0.01).
TABLE 1.
Vaccination of female BALB/c mice against E. multilocularis with recombinant EM95 antigen a
| Group | No. of mice | Level of infection b
|
Protection (%)c | |
|---|---|---|---|---|
| Mean (SE) | Range | |||
| Trial 1 | ||||
| PBS | 17 | 50.5 A (5.24) | 101-10 | |
| PBS + saponin | 15 | 50.5 B (5.4) | 84-15 | |
| GST + saponin | 13 | 39.5 C (6.4) | 103-16 | |
| EM95 + saponin | 14 | 8.5 D (1.42) | 21-2 | 78.5 |
| Trial 2 | ||||
| PBS | 15 | 24.1 E (4.16) | 63-7 | |
| PBS + saponin | 15 | 22.9 F (2.74) | 37-2 | |
| PBS + STP | 11 | 15.3 G (2.5) | 28-6 | |
| GST + saponin | 11 | 10.2 H (1.72) | 19-3 | |
| EM95 + STP | 11 | 5.6 I (1.18) | 13-1 | 63.1 |
| EM95 + saponin | 15 | 1.8 J (0.35) | 5-0 | 82.9 |
Each EM95- or GST-vaccinated mouse received three immunizations with 20 μg of EM95-GST fusion protein or control GST protein plus 50 μg of saponin or oil adjuvant (STP) 2 weeks apart. Two weeks after the second immunization, the mice were challenged orally with 3,000 E. multilocularis eggs, and the level of infection was assessed 6 weeks postinfection. Results of two independent trials are shown.
Statistical comparisons: A versus B, B versus C, E versus F, and E versus G, not significant; C versus D, P < 0.05; F versus H, G versus I, and H versus J, P < 0.01.
Level of protection was calculated as a percentage of the mean number of cysts in GST controls (for EM95 + saponin groups) or PBS-STP controls (for EM95 + STP groups).
The level of protection afforded by immunization with the EM95 protein (78.5 to 82.9%) is lower than that which has been obtained using the EG95 antigen in sheep (11, 12), although protection may be improved following optimization of such factors as the dose of antigen, number of immunizations, and route of immunization, etc. Nevertheless, the level of protection achieved has the potential to form the basis of the development of a practical vaccine which may be useful to reduce the level of alveolar echinococcosis in humans in regions of high endemicity.
Nucleotide sequence accession numbers.
The nucleotide sequence reported in this paper was deposited in the GenBank, EMBL, and DDBJ databases under accession numbers AY062920 and AY062921.
Acknowledgments
This work was funded with research grants from the National Health and Medical Research Council of Australia and the Grimminger Stiftung für Zoonoseforschung, Stuttgart, Germany.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byars, N. E., and A. C. Allison. 1987. Adjuvant formulation for use in vaccines to elicit both cell-mediated and humoral immunity. Vaccine 5:223-228. [DOI] [PubMed] [Google Scholar]
- 3.Chow, C., C. Gauci, A. F. Cowman, and M. W. Lightowlers. 2001. A gene family expressing a host-protective antigen of Echinococcus granulosus. Mol. Biochem. Parasitol. 118:83-88. [DOI] [PubMed] [Google Scholar]
- 4.Craig, P. S., P. Giraudoux, D. Shi, B. Bartholomot, G. Barnish, P. Delattre, J. P. Quere, S. Harraga, G. Bao, Y. Wang, F. Lu, A. Ito, and D. A. Vuitton. 2000. An epidemiological and ecological study of human alveolar echinococcosis transmission in south Gansu, China. Acta Trop. 77:167-177. [DOI] [PubMed] [Google Scholar]
- 5.Dayhoff, M. O. 1978. Atlas of Protein Sequence and Structure. National Biomedical Research Foundation, Silver Spring, Md.
- 6.Decker, C. J., and R. Parker. 1995. Diversity of cytoplasmic functions for the 3′ untranslated region of eukaryotic transcripts. Curr. Opin. Cell Biol. 7:386-392. [DOI] [PubMed] [Google Scholar]
- 7.Eckert, J., F. J. Conraths, and K. Tackmann. 2000. Echinococcosis: an emerging or re-emerging zoonosis? Int. J. Parasitol. 30:1283-1294. [DOI] [PubMed] [Google Scholar]
- 8.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 9.Lightowlers, M. W., A. Flisser, C. G. Gauci, D. D. Heath, O. Jensen, and R. Rolfe. 2000. Vaccination against cysticercosis and hydatid disease. Parasitol. Today 16:191-196. [DOI] [PubMed] [Google Scholar]
- 10.Lightowlers, M. W., and C. G. Gauci. 2001. Vaccines against cysticercosis and hydatidosis. Vet. Parasitol. 101:337-352. [DOI] [PubMed] [Google Scholar]
- 11.Lightowlers, M. W., O. Jensen, E. Fernandez, J. A. Iriarte, D. J. Woollard, C. G. Gauci, D. J. Jenkins, and D. D. Heath. 1999. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int. J. Parasitol. 29:531-534. [DOI] [PubMed] [Google Scholar]
- 12.Lightowlers, M. W., S. B. Lawrence, C. G. Gauci, J. Young, M. J. Ralston, D. Maas, and D. D. Health. 1996. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol. 18:457-462. [DOI] [PubMed] [Google Scholar]
- 13.Lightowlers, M. W., R. Rolfe, and C. G. Gauci. 1996. Taenia saginata: vaccination against cysticercosis in cattle with recombinant oncosphere antigens. Exp. Parasitol. 84:330-338. [DOI] [PubMed] [Google Scholar]
- 14.McManus, D. P., M. Knight, and A. J. Simpson. 1985. Isolation and characterisation of nucleic acids from the hydatid organisms, Echinococcus spp. (Cestoda). Mol. Biochem. Parasitol. 16:251-266. [DOI] [PubMed] [Google Scholar]
- 15.Muller, V. 1995. Ph.D. thesis. Studien zu einer rekombinanten Vakzine gegen Echinococcus multilocularis im Mausmodell. University of Hohenheim, Stuttgart, Germany.
- 16.Nakai, K., and M. Kanehisa. 1992. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 14:897-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponting, C. P., J. Schultz, F. Milpetz, and P. Bork. 1999. SMART: identification and annotation of domains from signalling and extracellular protein sequences. Nucleic Acids Res. 27:229-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romig, T., Bilger, B., Merli, M., Dinkel, A., Lucius, R., and Mackenstedt, U. 1999. Bekämpfung von Echinococcus multilocularis in einem Hochendemiegebiet Süddeutschlands, p. 172-183. Tagung der Fachgruppe Parasitologie und parasitäre Krankheiten. Deutsche Veterinärmedizinische Gesellschaft, Hannover, Germany.
- 19.Schantz, J., J. Chai, P. S. Craig, J. Eckert, D. J. Jenkins, C. N. L. Macpherson, and A. Thakur. 1995. Epidemiology and control of hydatid disease, p. 233-331. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 20.Senapathy, P., M. B. Shapiro, and N. L. Harris. 1990. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 183:252-278. [DOI] [PubMed] [Google Scholar]
- 21.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson, R. C. A., and A. J. Lymbery (ed.). 1995. Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 24.Waterkeyn, J., C. Gauci, A. Cowman, and M. Lightowlers. 1997. Sequence analysis of a gene family encoding Taenia ovis vaccine antigens expressed during embryogenesis of eggs. Mol. Biochem. Parasitol. 86:75-84. [DOI] [PubMed] [Google Scholar]
- 25.Waterkeyn, J. G., M. W. Lightowlers, R. Coppel, and A. F. Cowman. 1995. Characterization of the gene family encoding a host-protective antigen of the tapeworm Taenia ovis. Mol. Biochem. Parasitol. 73:123-131. [DOI] [PubMed] [Google Scholar]
- 26.Woollard, D. J., C. G. Gauci, D. D. Heath, and M. W. Lightowlers. 1998. Epitope specificities and antibody responses to the EG95 hydatid vaccine. Parasite Immunol. 20:535-540. [DOI] [PubMed] [Google Scholar]