Abstract
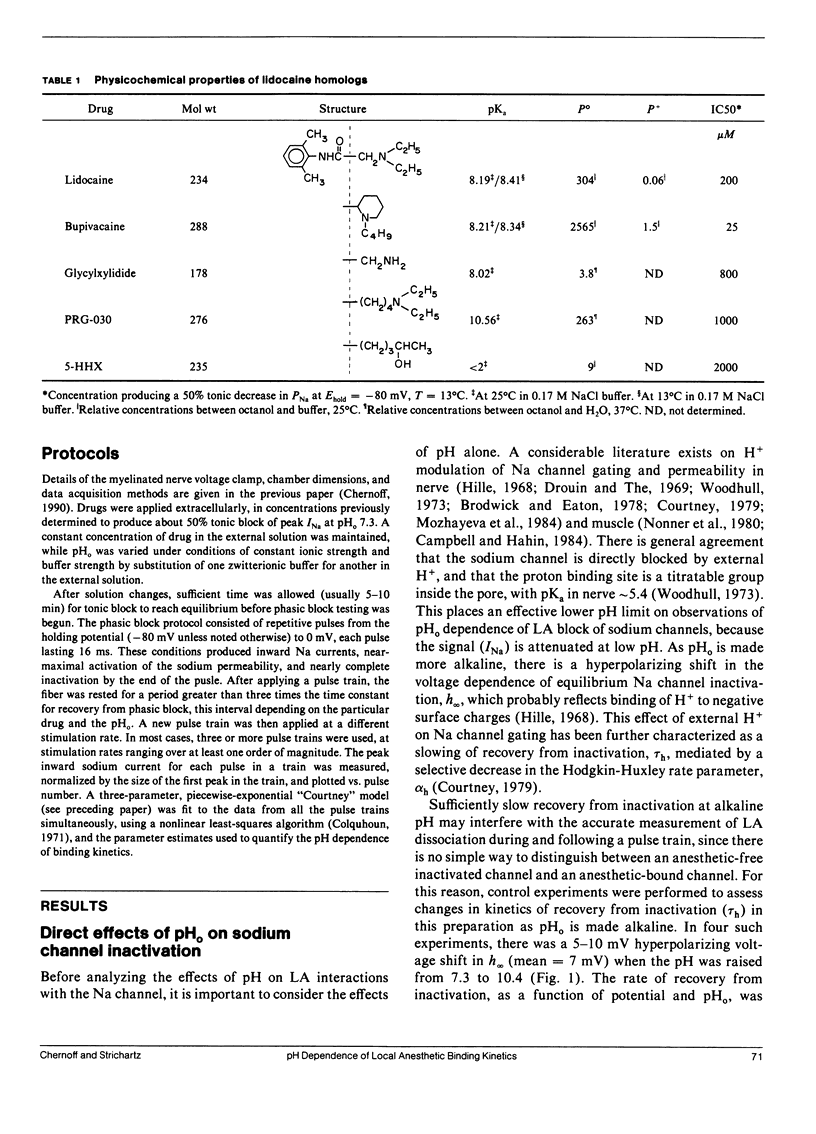
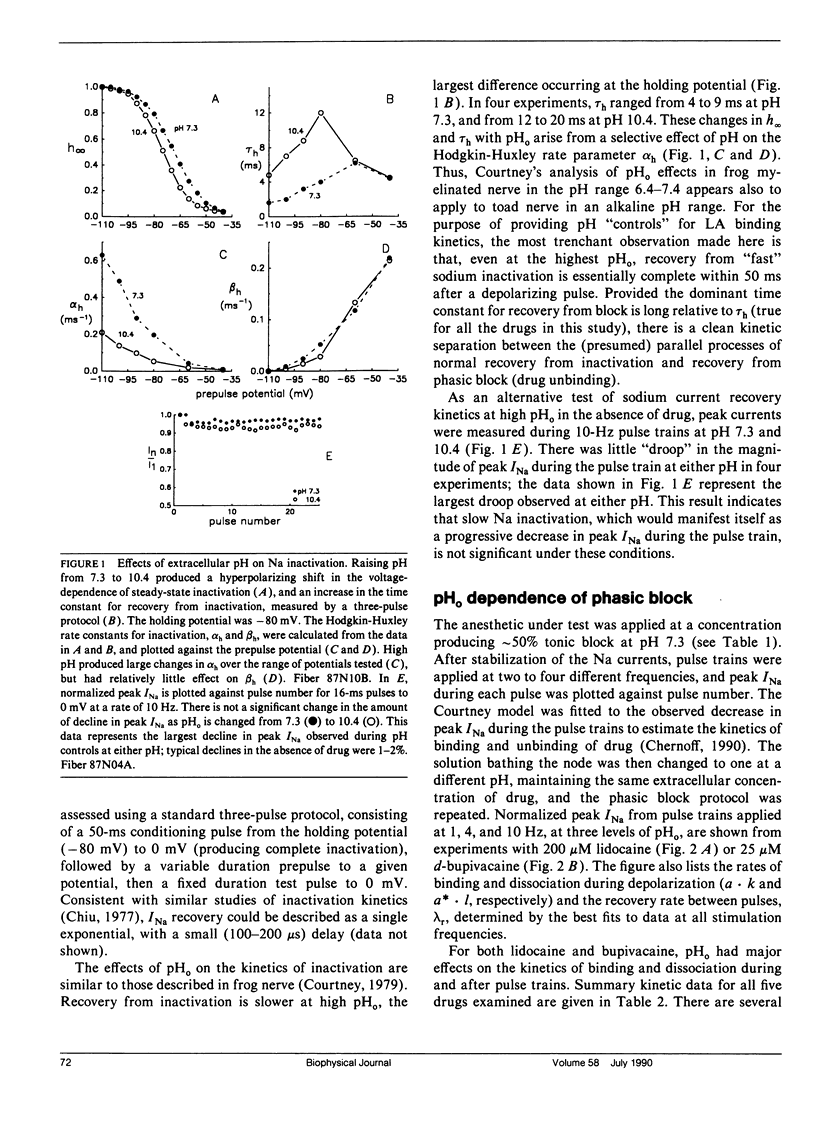
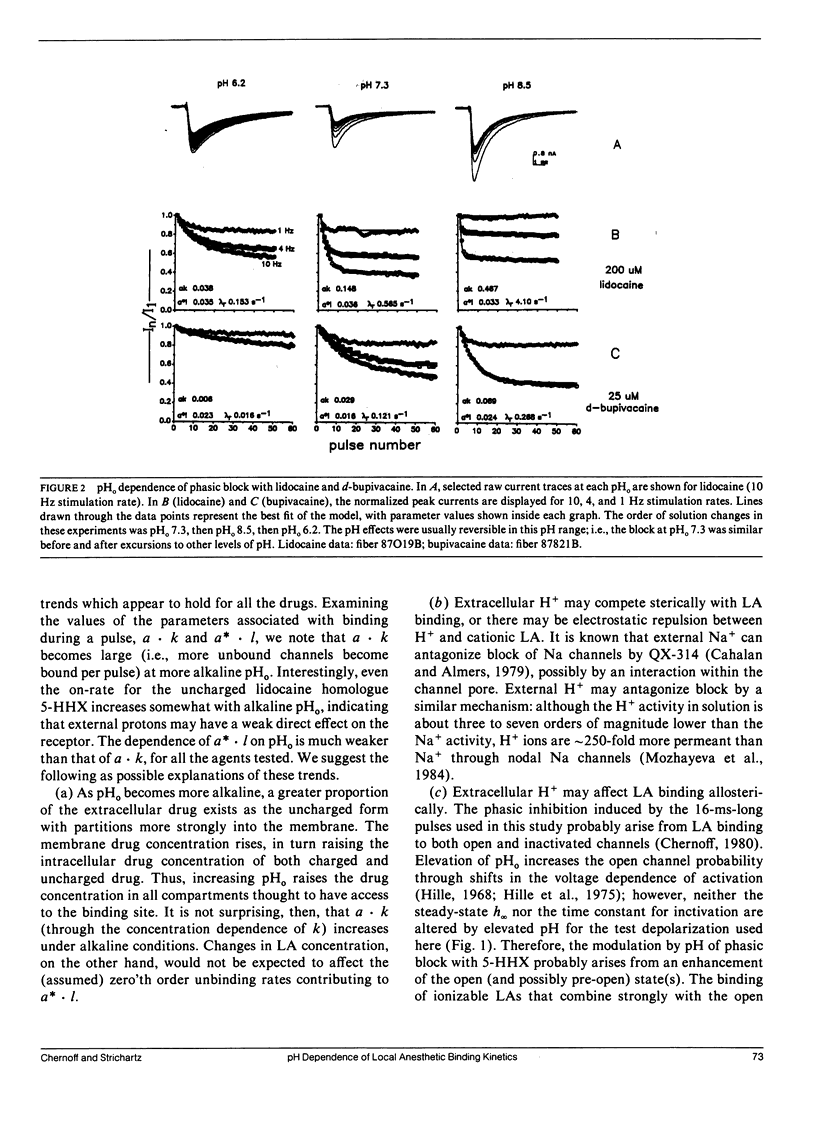
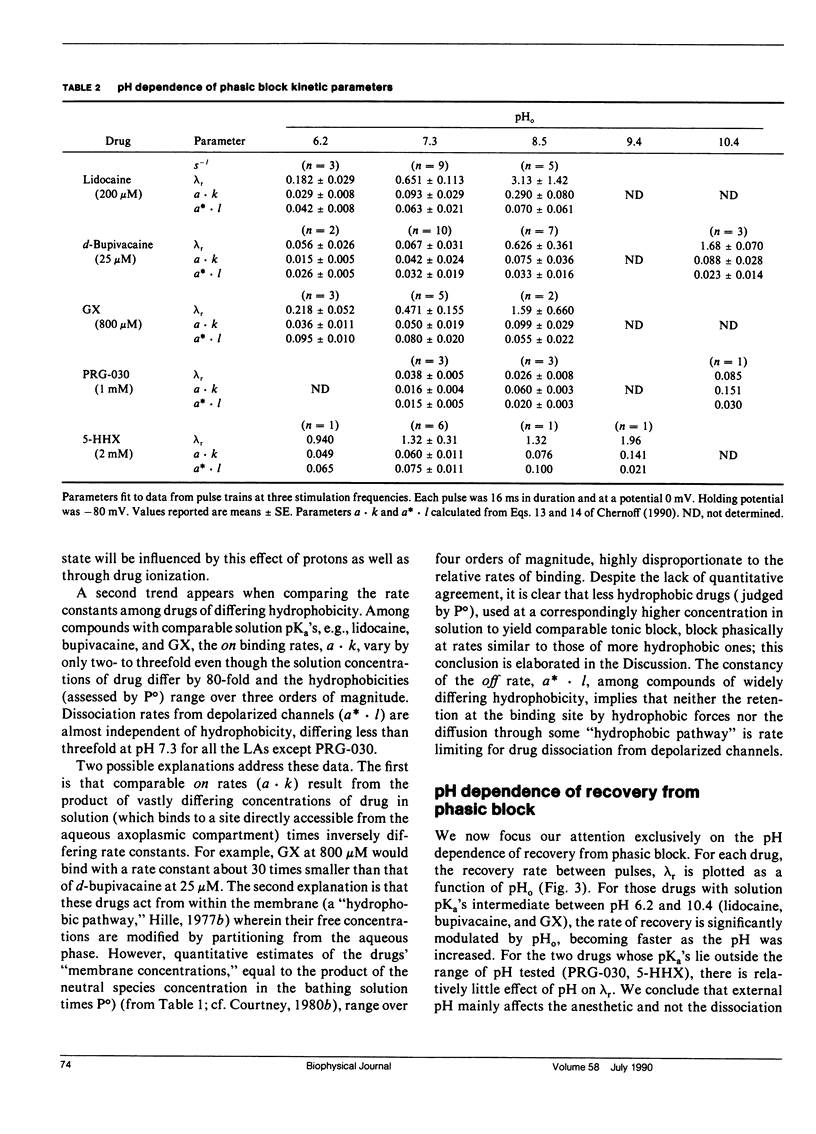
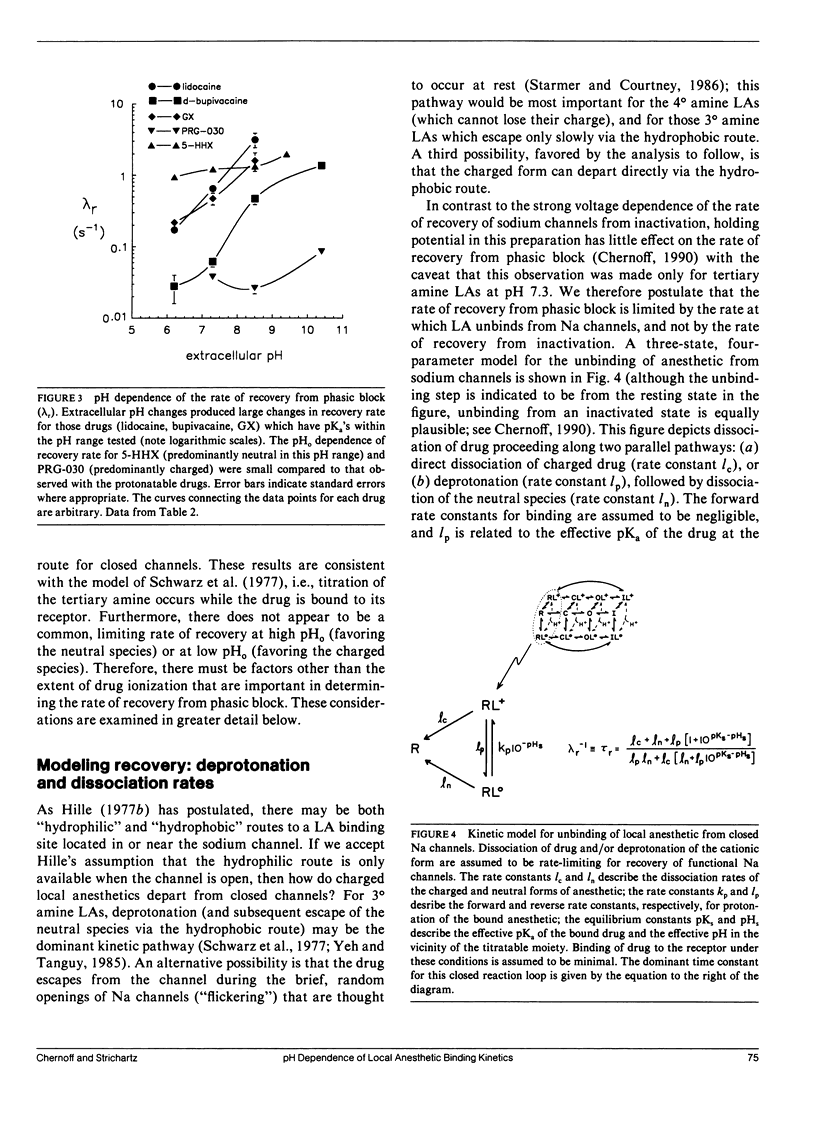
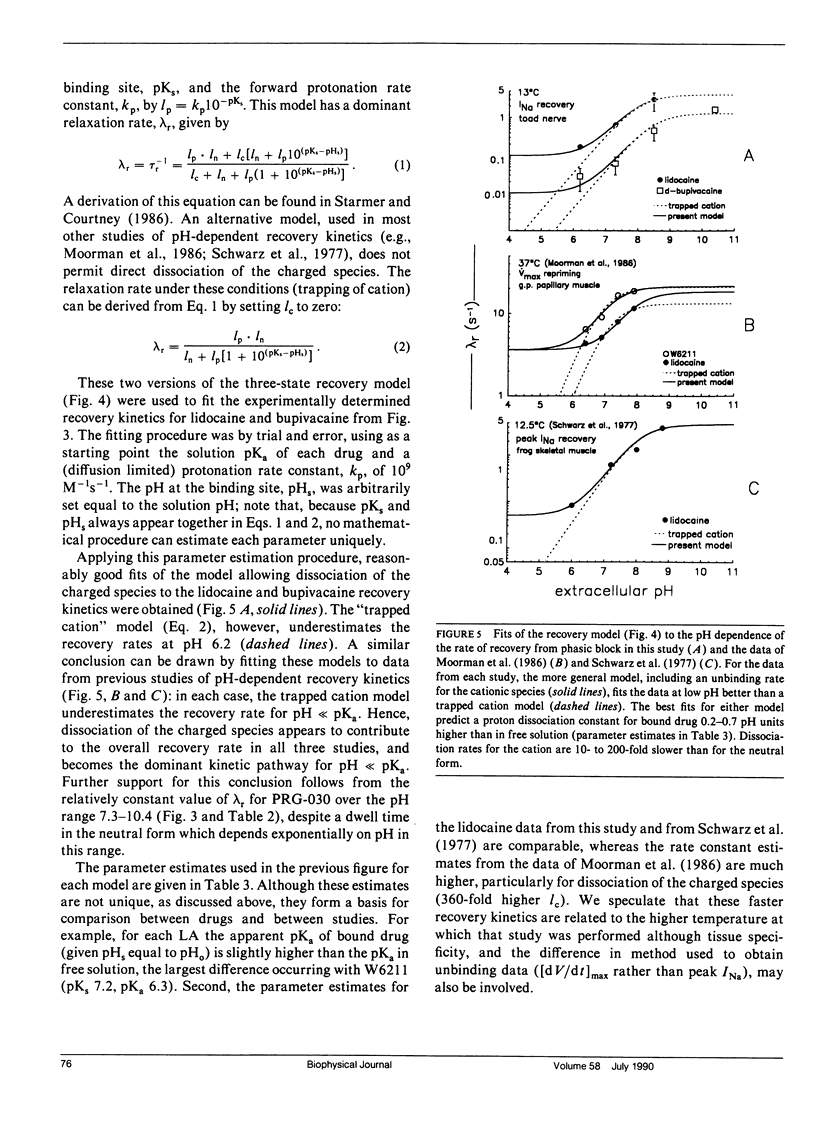
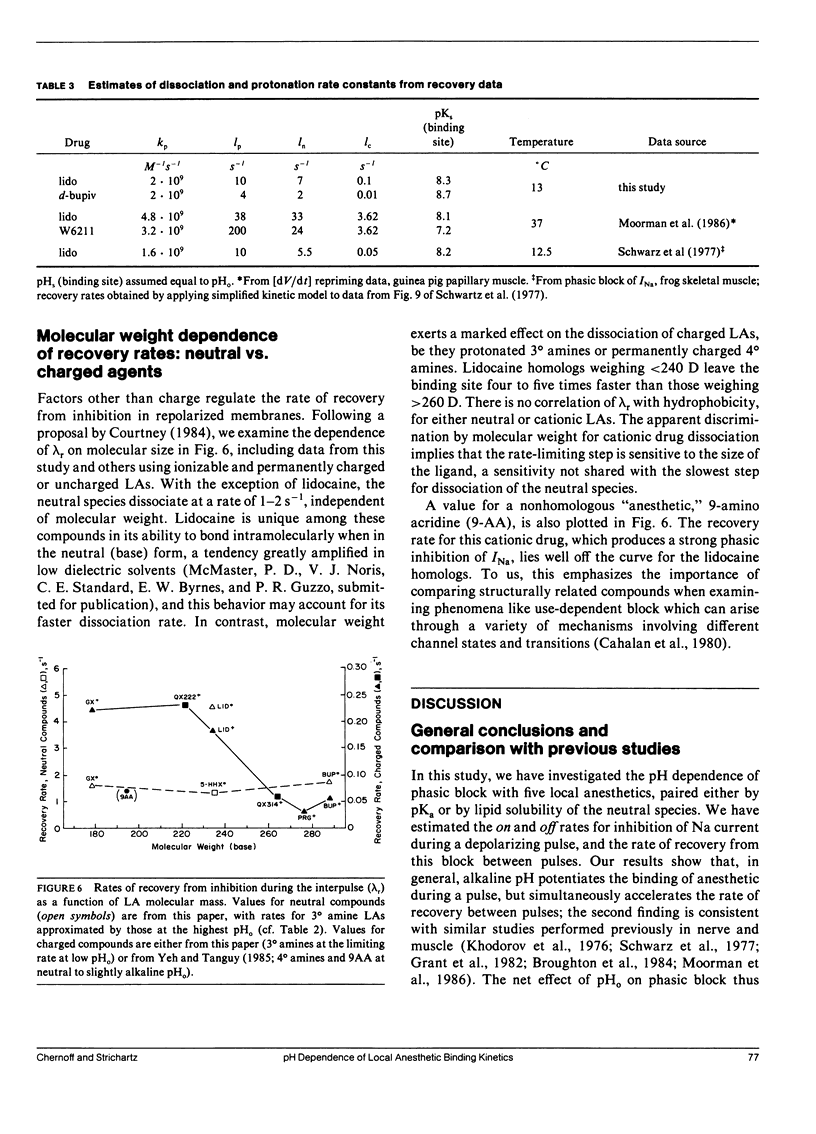
This study assesses the importance of local anesthetic charge and hydrophobicity in determining the rates of binding to and dissociation from neuronal Na channels. Five amide-linked local anesthetics, paired either by similar pKa or hydrophobicity, were chosen for study: lidocaine, two tertiary amine lidocaine homologs, a neutral lidocaine homolog, and bupivacaine. Voltage-clamped nodes of Ranvier from the sciatic nerve of Bufo marinus were exposed to anesthetic externally, and use-dependent ("phasic") block of Na current was observed. Kinetic analysis of binding (blocking) rates was performed using a three parameter, piecewise-exponential binding model. Changes in extracellular pH (pHo) were used to assess the role of drug protonation in determining the rate of onset of, and recovery from, phasic block. For those drugs with pKa's in the range of pHo tested (6.2-10.4), the forward binding rate during a depolarizing pulse increased at higher pH, consistent with an increase in either intracellular or intramembrane concentration of drug. The rate for unbinding during depolarization was independent of pHo. The dissociation rate between pulses also increased at higher pHo. The pHo dependence of the dissociation rate was not consistent with a model in which the cation is trapped relentlessly within a closed channel. Quantitative estimates of dissociation rates show that the cationic form of lidocaine dissociates at a rate of 0.1 s-1 (at 13 degrees C); for neutral lidocaine, the dissociation rate is 7.0 s-1. Furthermore, the apparent pKa of bound local anesthetic was found to be close to the pKa in aqueous solution, but different than the pKa for "free" local anesthetic accessible to the depolarized channel.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulanger Y., Schreier S., Smith I. C. Molecular details of anesthetic--lipid interaction as seen by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1981 Nov 24;20(24):6824–6830. doi: 10.1021/bi00527a013. [DOI] [PubMed] [Google Scholar]
- Brodwick M. S., Eaton D. C. Sodium channel inactivation in squid axon is removed by high internal pH or tyrosine-specific reagents. Science. 1978 Jun 30;200(4349):1494–1496. doi: 10.1126/science.26973. [DOI] [PubMed] [Google Scholar]
- Broughton A., Grant A. O., Starmer C. F., Klinger J. K., Stambler B. S., Strauss H. C. Lipid solubility modulates pH potentiation of local anesthetic block of Vmax reactivation in guinea pig myocardium. Circ Res. 1984 Oct;55(4):513–523. doi: 10.1161/01.res.55.4.513. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979 Jul;27(1):39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T., Hahin R. Altered sodium and gating current kinetics in frog skeletal muscle caused by low external pH. J Gen Physiol. 1984 Nov;84(5):771–788. doi: 10.1085/jgp.84.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff D. M. Kinetic analysis of phasic inhibition of neuronal sodium currents by lidocaine and bupivacaine. Biophys J. 1990 Jul;58(1):53–68. doi: 10.1016/S0006-3495(90)82353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff D. M., Strichartz G. R. Tonic and phasic block of neuronal sodium currents by 5-hydroxyhexano-2',6'-xylide, a neutral lidocaine homologue. J Gen Physiol. 1989 Jun;93(6):1075–1090. doi: 10.1085/jgp.93.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson C. W., Hondeghem L. M. Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology. 1985 Apr;62(4):396–405. [PubMed] [Google Scholar]
- Courtney K. R. Extracellular pH selectively modulates recovery from sodium inactivation in frog myelinated nerve. Biophys J. 1979 Nov;28(2):363–368. doi: 10.1016/S0006-3495(79)85183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R. Interval-dependent effects of small antiarrhythmic drugs on excitability of guinea-pig myocardium. J Mol Cell Cardiol. 1980 Nov;12(11):1273–1286. doi: 10.1016/0022-2828(80)90071-1. [DOI] [PubMed] [Google Scholar]
- Courtney K. R., Kendig J. J., Cohen E. N. The rates of interaction of local anesthetics with sodium channels in nerve. J Pharmacol Exp Ther. 1978 Nov;207(2):594–604. [PubMed] [Google Scholar]
- Courtney K. R. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975 Nov;195(2):225–236. [PubMed] [Google Scholar]
- Courtney K. R. Quantitative structure/activity relations based on use-dependent block and repriming kinetics in myocardium. J Mol Cell Cardiol. 1987 Mar;19(3):319–330. doi: 10.1016/s0022-2828(87)80599-0. [DOI] [PubMed] [Google Scholar]
- Courtney K. R. Size-dependent kinetics associated with drug block of sodium current. Biophys J. 1984 Jan;45(1):42–44. doi: 10.1016/S0006-3495(84)84100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R. Structure-activity relations for frequency-dependent sodium channel block in nerve by local anesthetics. J Pharmacol Exp Ther. 1980 Apr;213(1):114–119. [PubMed] [Google Scholar]
- Drouin H., The R. The effect of reducing extracellular pH on the membrane currents of the ranvier node. Pflugers Arch. 1969;313(1):80–88. doi: 10.1007/BF00586331. [DOI] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Frazier D. T., Narahashi T., Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):45–51. [PubMed] [Google Scholar]
- Grant A. O., Strauss L. J., Wallace A. G., Strauss H. C. The influence of pH on th electrophysiological effects of lidocaine in guinea pig ventricular myocardium. Circ Res. 1980 Oct;47(4):542–550. doi: 10.1161/01.res.47.4.542. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Trantham J. L., Brown K. K., Strauss H. C. PH-Dependent effects of quinidine on the kinetics of dV/dtmax in guinea pig ventricular myocardium. Circ Res. 1982 Feb;50(2):210–217. doi: 10.1161/01.res.50.2.210. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977 Apr;69(4):475–496. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Yee R., Bjornsson T., Starmer C. F., Grant A. O., Strauss H. C. pKa does not predict pH potentiation of sodium channel blockade by lidocaine and W6211 in guinea pig ventricular myocardium. J Pharmacol Exp Ther. 1986 Jul;238(1):159–166. [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P., Nosyreva E. D. A study on the potential-dependence of proton block of sodium channels. Biochim Biophys Acta. 1984 Sep 5;775(3):435–440. doi: 10.1016/0005-2736(84)90201-3. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Frazier T., Yamada M. The site of action and active form of local anesthetics. I. Theory and pH experiments with tertiary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):32–44. [PubMed] [Google Scholar]
- Nonner W., Spalding B. C., Hille B. Low intracellular pH and chemical agents slow inactivation gating in sodium channels of muscle. Nature. 1980 Mar 27;284(5754):360–363. doi: 10.1038/284360a0. [DOI] [PubMed] [Google Scholar]
- Schreier S., Frezzatti W. A., Jr, Araujo P. S., Chaimovich H., Cuccovia I. M. Effect of lipid membranes on the apparent pK of the local anesthetic tetracaine. Spin label and titration studies. Biochim Biophys Acta. 1984 Jan 11;769(1):231–237. doi: 10.1016/0005-2736(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Palade P. T., Hille B. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J. 1977 Dec;20(3):343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C. F., Courtney K. R. Modeling ion channel blockade at guarded binding sites: application to tertiary drugs. Am J Physiol. 1986 Oct;251(4 Pt 2):H848–H856. doi: 10.1152/ajpheart.1986.251.4.H848. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O., Strauss H. C. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys J. 1984 Jul;46(1):15–27. doi: 10.1016/S0006-3495(84)83994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel G. E., Bianchi C. P. The effects of pH gradients on the action of procaine and lidocaine in intact and desheathed sciatic nerves. J Pharmacol Exp Ther. 1970 Mar;172(1):1–17. [PubMed] [Google Scholar]
- Tenthorey P. A., Block A. J., Ronfeld R. A., McMaster P. D., Byrnes E. W. New antiarrhythmic agents. 6. Quantitative structure-activity relationships of aminoxylidides. J Med Chem. 1981 Jul;24(7):798–806. doi: 10.1021/jm00139a007. [DOI] [PubMed] [Google Scholar]
- Watts A., Poile T. W. Direct determination by 2H-NMR of the ionization state of phospholipids and of a local anaesthetic at the membrane surface. Biochim Biophys Acta. 1986 Oct 9;861(2):368–372. doi: 10.1016/0005-2736(86)90440-2. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Tanguy J. Na channel activation gate modulates slow recovery from use-dependent block by local anesthetics in squid giant axons. Biophys J. 1985 May;47(5):685–694. doi: 10.1016/S0006-3495(85)83965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., TenEick R. E. Molecular and structural basis of resting and use-dependent block of sodium current defined using disopyramide analogues. Biophys J. 1987 Jan;51(1):123–135. doi: 10.1016/S0006-3495(87)83317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


