Abstract
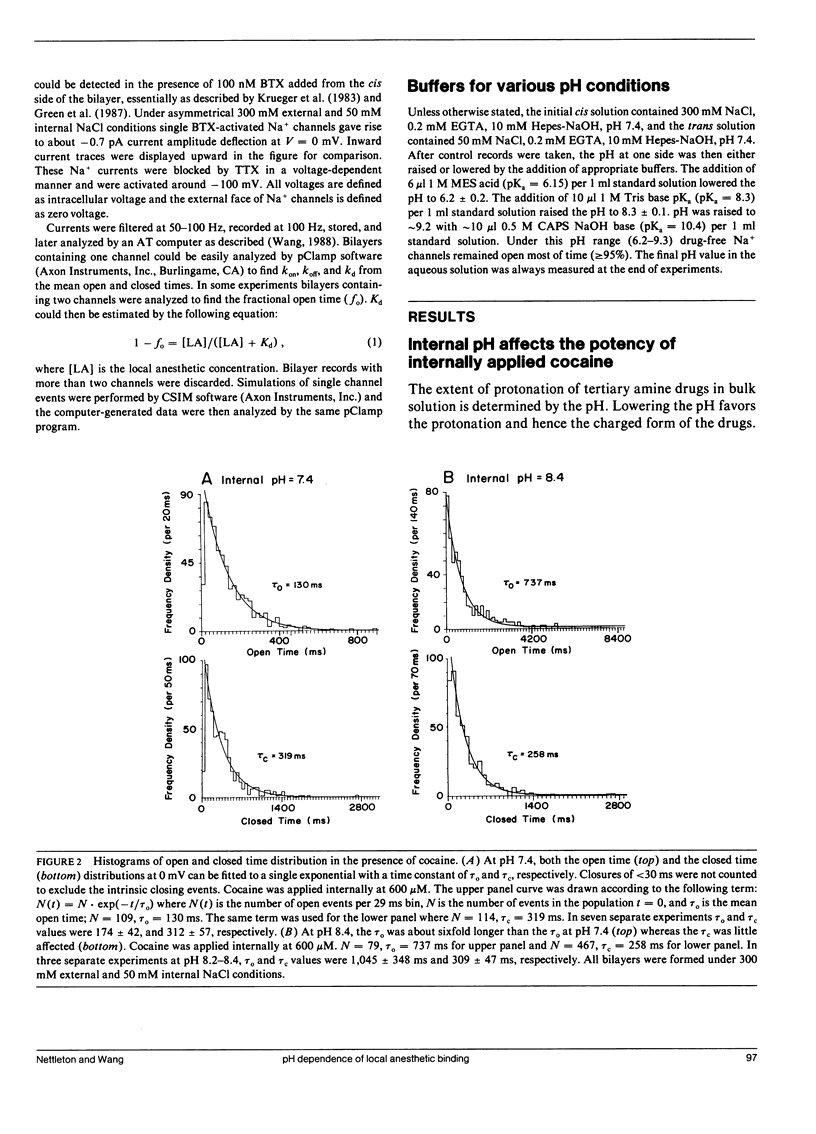
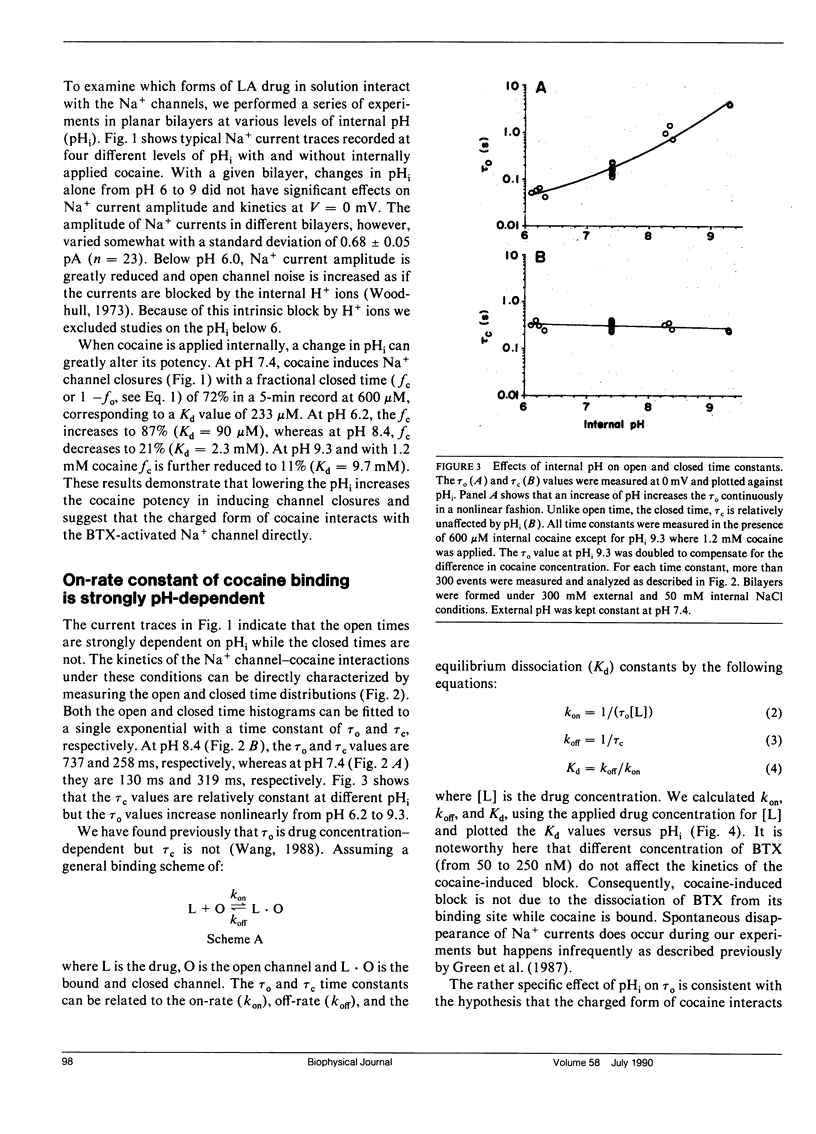
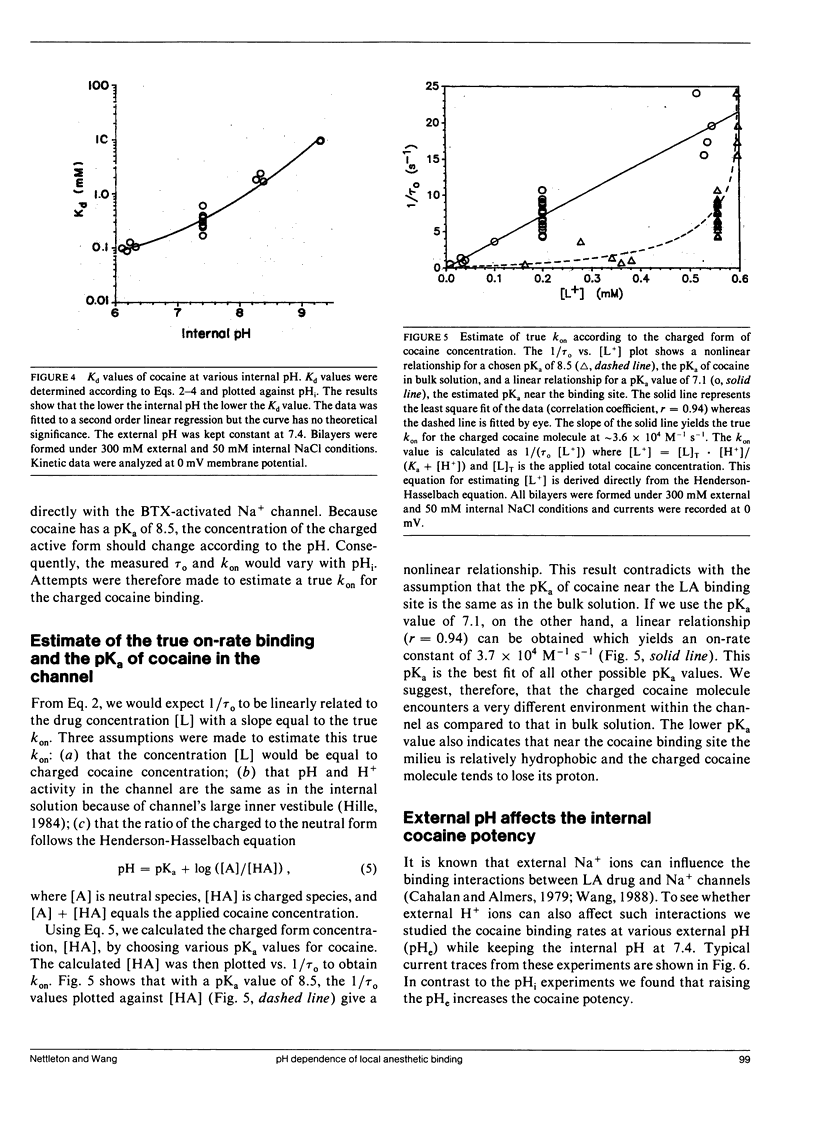
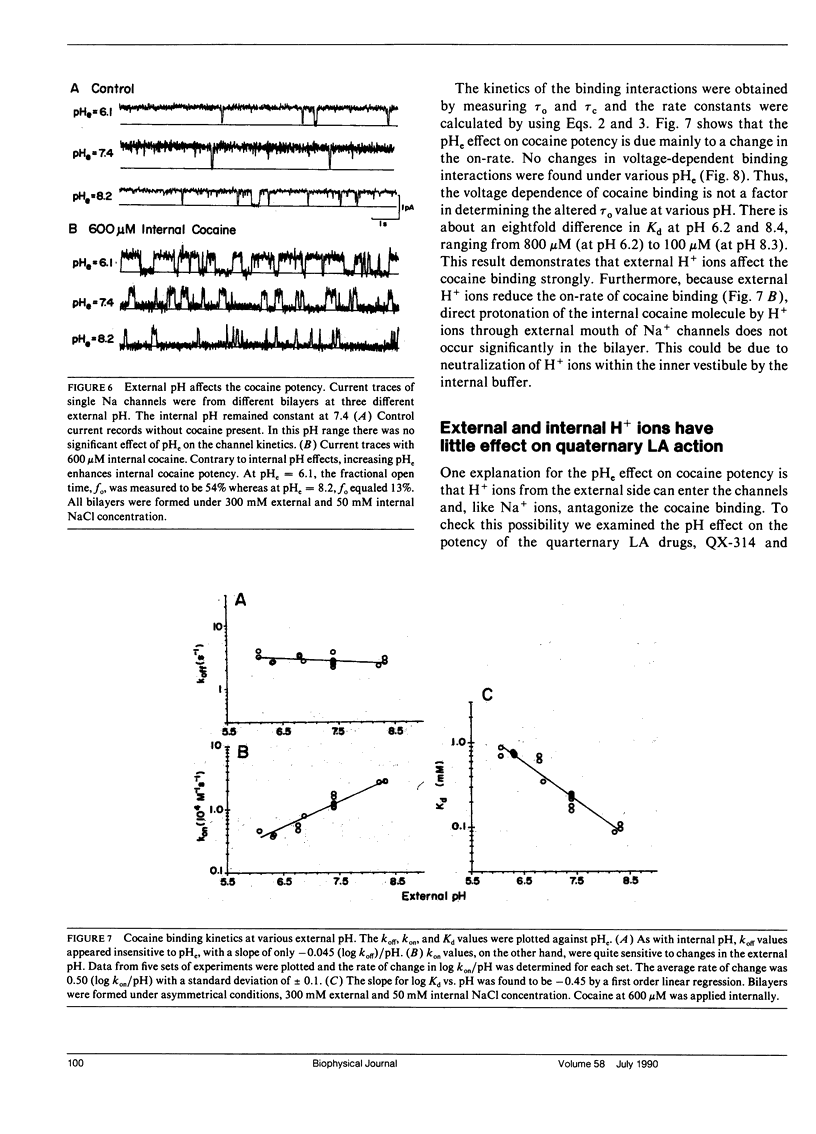
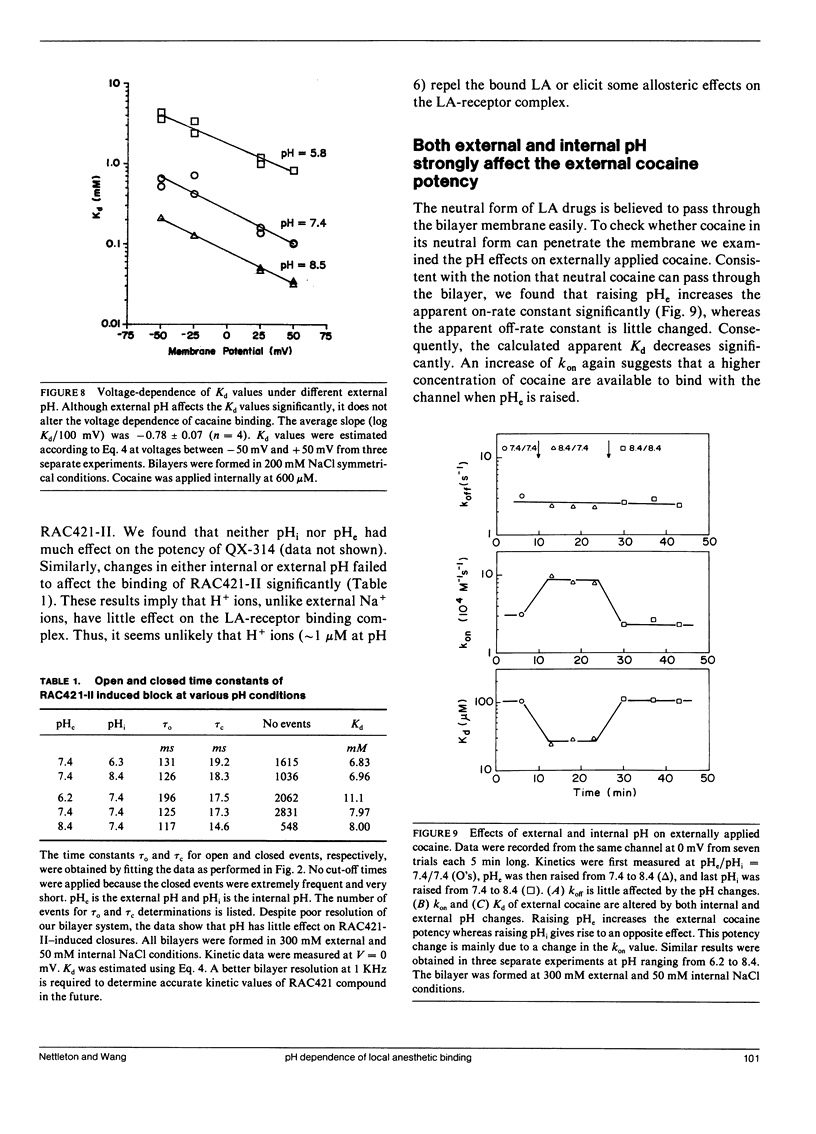
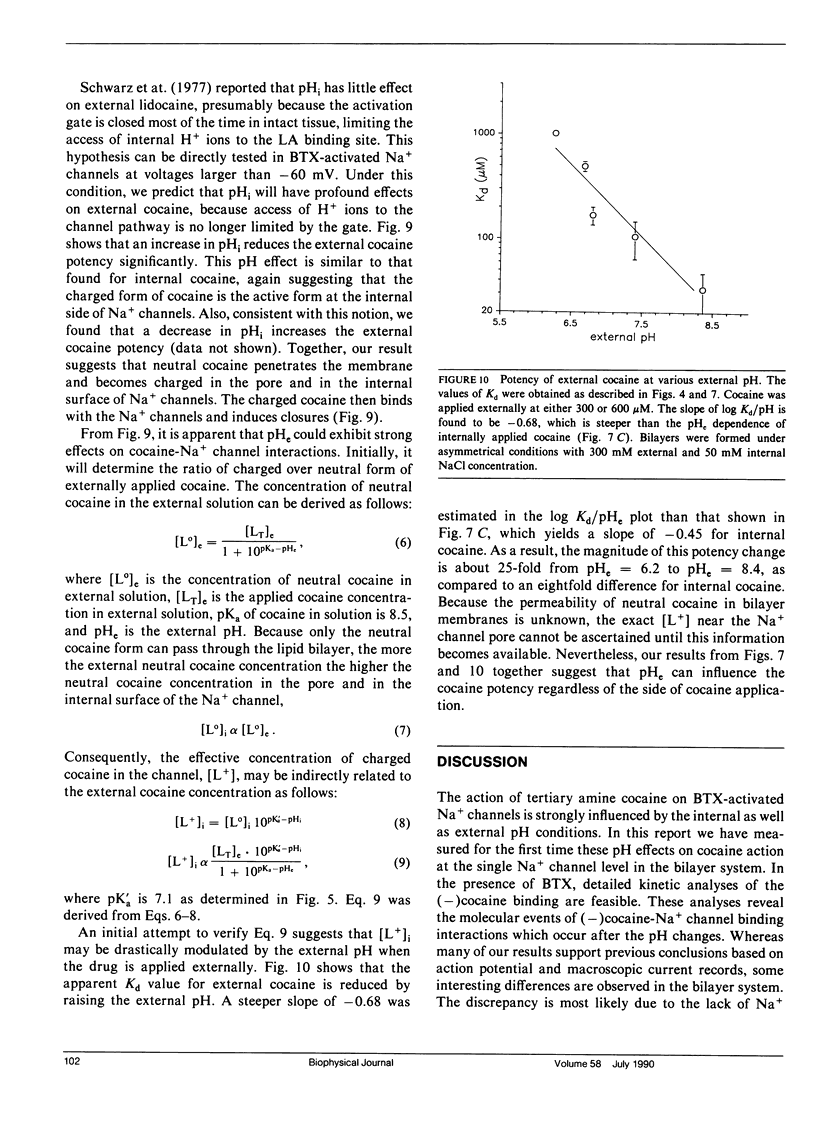
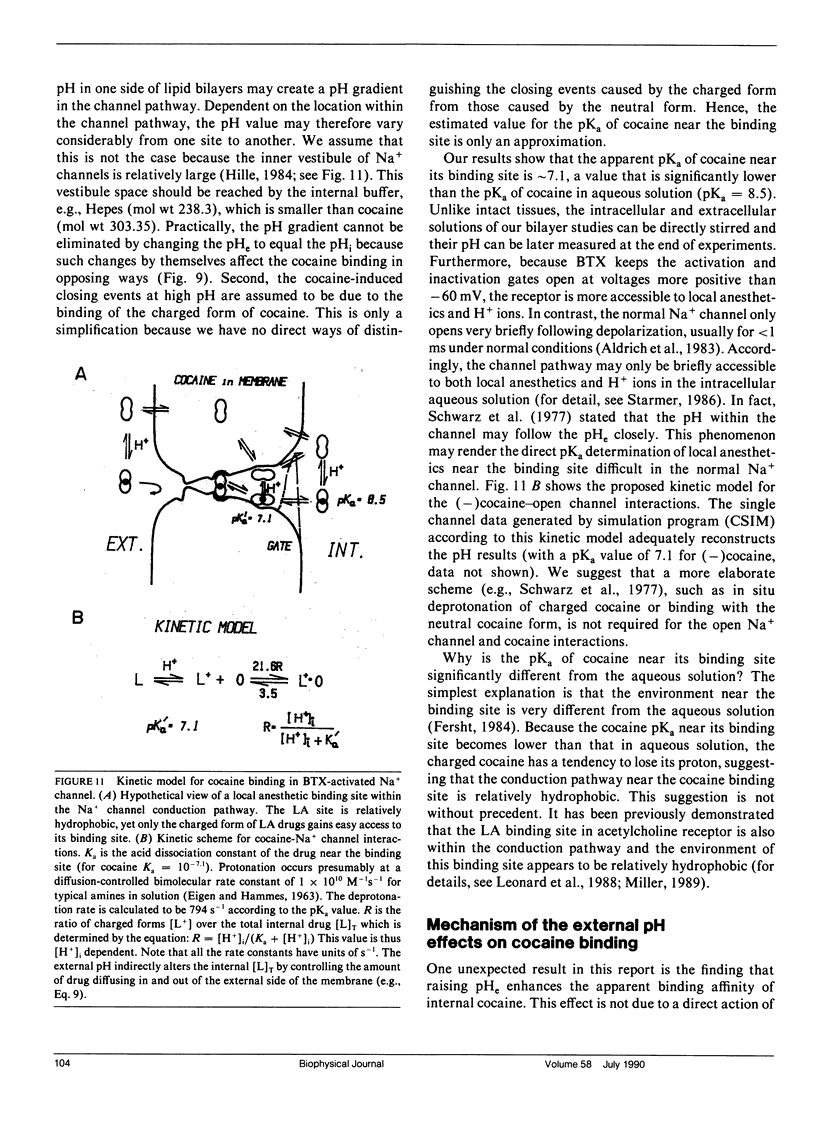
The effects of internal and external pH on the binding kinetics of local anesthetics (LAs) were studied in single batrachotoxin-activated Na+ channels incorporated into planar bilayers. With internal quaternary QX-314 and RAC421-II drugs, the binding interactions were little affected by either external or internal pH. With tertiary cocaine, the binding kinetics were drastically altered by pH. A decrease in the internal pH from 9.3 to 6.2 decreased the apparent equilibrium dissociation constant (Kd) of internal cocaine by more than 100-fold. This increase in the binding affinity was mostly accounted for by an increase in the apparent cocaine on-rate constant (kon) of approximately 80-fold. The cocaine off-rate constant (koff) was little changed (between 3-4 s-1). These results demonstrate quantitatively that the charged form of cocaine is the active form for BTX-activated Na+ channels. Surprisingly, the apparent pKa of cocaine near its binding site was estimated to be 1.4 units lower than that in bulk solution (7.1 vs. 8.5), indicating that the LA drug encounters a relatively hydrophobic environment. Opposite to the internal pH effect, a decrease of external pH from 8.4 to 6.2 increased the Kd value of internally and externally applied cocaine by approximately 8- and approximately 25-fold, respectively. External pH effect was primarily mediated by modulation of kon; koff was again relatively unaffected. Our findings support a model in which neutral cocaine can readily cross the membrane barrier, but needs to be protonated internally to bind to its binding site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979 Jul;27(1):39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettbarn W. D., Heilbronn E., Hoskin F. C., Kitz R. The effects of pH on penetration and action of procaine 14C, atropine 3H, n-butanol 14C and halothane 14C in single giant axons of the squid. Neuropharmacology. 1972 Sep;11(5):727–732. doi: 10.1016/0028-3908(72)90082-2. [DOI] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Frazier D. T., Narahashi T., Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):45–51. [PubMed] [Google Scholar]
- Green W. N., Weiss L. B., Andersen O. S. Batrachotoxin-modified sodium channels in planar lipid bilayers. Ion permeation and block. J Gen Physiol. 1987 Jun;89(6):841–872. doi: 10.1085/jgp.89.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Worley J. F., 3rd, French R. J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature. 1983 May 12;303(5913):172–175. doi: 10.1038/303172a0. [DOI] [PubMed] [Google Scholar]
- Miller C. Genetic manipulation of ion channels: a new approach to structure and mechanism. Neuron. 1989 Mar;2(3):1195–1205. doi: 10.1016/0896-6273(89)90304-8. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E. G., Latorre R. Saxitoxin and ouabain binding activity of isolated skeletal muscle membrane as indicators of surface origin and purity. Biochim Biophys Acta. 1983 Jul 27;732(2):412–420. doi: 10.1016/0005-2736(83)90058-5. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Garber S. S., Miller C. Batrachotoxin-activated Na+ channels in planar lipid bilayers. Competition of tetrodotoxin block by Na+. J Gen Physiol. 1984 Nov;84(5):665–686. doi: 10.1085/jgp.84.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Uehara A., Guo X., Heiny J. Isochannels and blocking modes of voltage-dependent sodium channels. Ann N Y Acad Sci. 1986;479:269–292. doi: 10.1111/j.1749-6632.1986.tb15575.x. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Yee R., Bjornsson T., Starmer C. F., Grant A. O., Strauss H. C. pKa does not predict pH potentiation of sodium channel blockade by lidocaine and W6211 in guinea pig ventricular myocardium. J Pharmacol Exp Ther. 1986 Jul;238(1):159–166. [PubMed] [Google Scholar]
- Narahashi T., Frazier T., Yamada M. The site of action and active form of local anesthetics. I. Theory and pH experiments with tertiary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):32–44. [PubMed] [Google Scholar]
- Schwarz W., Palade P. T., Hille B. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J. 1977 Dec;20(3):343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C. F. Theoretical characterization of ion channel blockade: ligand binding to periodically accessible receptors. J Theor Biol. 1986 Mar 21;119(2):235–249. doi: 10.1016/s0022-5193(86)80077-7. [DOI] [PubMed] [Google Scholar]
- Wang G. K. Cocaine-induced closures of single batrachotoxin-activated Na+ channels in planar lipid bilayers. J Gen Physiol. 1988 Dec;92(6):747–765. doi: 10.1085/jgp.92.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]



