Abstract
Helminth infections induce Th2-type biased immune responses. Although the mechanisms involved in this phenomenon are not yet clearly defined, antigen-presenting cells (APC) could play an important role in this process. Here, we have used peritoneal macrophages (F4/80+) recruited at different times after challenge with Taenia crassiceps as APC and tested their ability to regulate Th1/Th2 differentiation. Macrophages from acute infections produced high levels of interleukin-12 (IL-12) and nitric oxide (NO), paralleled with low levels of IL-6 and prostaglandin E2 (PGE2) and with the ability to induce strong antigen-specific CD4+ T-cell proliferation in response to nonrelated antigens. In contrast, macrophages from chronic infections produced higher levels of IL-6 and PGE2 and had suppressed production of IL-12 and NO, associated with a poor ability to induce antigen-specific proliferation in CD4+ T cells. Failure to induce proliferation was not due to a deficient expression of accessory molecules, since major histocompatibility complex class II, CD40, and B7-2 were up-regulated, together with CD23 and CCR5 as infection progressed. These macrophages from chronic infections were able to bias CD4+ T cells to produce IL-4 but not gamma interferon (IFN-γ), contrary to macrophages from acute infections. Blockade of B7-2 and IL-6 and inhibition of PGE2 failed to restore the proliferative response in CD4+ T cells. Furthermore, studies using STAT6−/− mice revealed that STAT6-mediated signaling was essential for the expansion of these alternatively activated macrophages. These data demonstrate that helminth infections can induce different macrophage populations that have Th2-biasing properties.
After stimulation, CD4+ T cells differentiate into distinct subsets characterized by their functions and their cytokine profiles (Th1 or Th2 cells). Th1 cells produce high levels of interleukin 2 (IL-2) and gamma interferon (IFN-γ), which are strong inducers of cell-mediated immunity, whereas Th2 cells produce cytokines such as IL-4, IL-5, IL-6, IL-10, and IL-13, which provide signals for B-cell activation and antibody production (34). Although the mechanisms that generate both subsets have been studied in detail, there is not enough convincing evidence of one isolated process inducing polarization of CD4 T cells towards a Th1 or Th2 outcome. Nevertheless, several factors, such as the major histocompatibility complex (MHC) haplotype, dose and nature of the antigen, route of antigen administration, costimulatory molecules, cytokine microenvironment, and type of antigen-producing cells (APC) (2, R6, R7, 24), have been shown to play a role in the polarization of the immune response. Since it is possible that most in vivo responses do not take place in a milieu with adequate levels of cytokines to stimulate T cells, professional APC may play an important role in Th differentiation by secreting the essential cytokines locally and providing the ligands for T-cell-receptor and costimulatory signals. Thus, the state of activation of APC could be a decisive factor inducing the polarization of the immune response.
A key feature of helminth infections is the induction of strong Th2-biased immune responses in their hosts. Why and how helminths induce Th2-type biased responses are still unresolved questions. It has been suggested that excreted/secreted products from helminth parasites play an important role in these Th2 responses (16, 36). However, the precise mechanism of action or the target cell for these products is still uncertain. Indeed, parasitic helminth infections can modify the normal host immune response against another nonrelated antigenic stimulus (8, 21) and/or also modify the susceptibility to others parasites (15, 38) or viruses (1), suggesting an important influence of helminth infections in the immunological micro-environment.
Also, experimental murine cysticercosis caused by the helminth Taenia crassiceps induces polarized type 2 cytokine profiles; another key feature is a gradual shifting from an initial restrictive Th1-type response to a late permissive Th2-type response in the infected host (41, 45). We also have previously reported that infection with T. crassiceps cysticerci renders their host more resistant or more susceptible to a secondary nonrelated infection depending on the time course of T. crassiceps implantation (38). A series of recent studies suggest that a subset of macrophages, called alternatively activated, plays a role in the Th2 biased response induced in nematode infections (4, 25, 26, 28). Therefore, we hypothesized that these alternatively activated macrophages can be a more general mechanism to down-modulate T-cell proliferation and favor Th2 development in helminth infections. To test this hypothesis, we isolated peritoneal macrophages from T. crassiceps-infected mice during acute as well as chronic infection and used them as APC to determine whether they induce preferential Th2 differentiation.
We found that macrophages obtained during chronic infections showed a poor ability to induce proliferative responses in CD4+ T cells when used as APC but favored IL-4 production. Furthermore, these cells expressed high levels of both CD23 and CCR5, which were associated with low IL-12 and nitric oxide (NO) production, but secreted high levels of IL-6 and prostaglandin E2 (PGE2). This contrasted with macrophages recruited early during infections, which induced stronger CD4+-T-cell proliferation and high levels of IFN-γ. Our data indicate that the state of activation of APC induced by a chronic helminth infection modulates the final pattern of response of CD4+ T cells previously sensitized with a nonrelated parasite antigen. This is a possible mechanism for Th2 biased responses in helminth infections.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and were maintained in a pathogen-free environment at the Harvard Medical School animal facility in accordance with institutional guidelines. In some experiments STAT6-KO mice in a BALB/c background (Jackson Labs) were used.
Parasites and infections.
Metacestodes of T. crassiceps (ORF strain) were harvested from the peritoneal cavity of female BALB/c mice after 2 to 4 months of infection. The cysticerci were washed four times in sterile phosphate-buffered saline (PBS) (0.15 M, pH 7.2). Experimental infection was achieved by intraperitoneal (i.p.) injection with 20 small (diameter, ca. 2 mm) nonbudding cysticerci of T. crassiceps suspended in 0.3 ml of PBS per mouse, and infections were done at different times in order to perform all assays at the same day, using age-matched uninfected mice as controls for each time point.
Peritoneal macrophage extraction and pattern of response to LPS.
Peritoneal exudate cells (PECs) were obtained from the peritoneal cavities of mice infected with T. crassiceps for 2, 4, 8, and 12 weeks or from uninfected mice injected with thioglycolate (3%) 4 days earlier. The cells were washed twice with Hanks' solution, and erythrocytes were lysed by resuspending cells in Boyle's solution (0.17 M Tris and 0.16 M ammonium chloride). Following two washes, viable cells were counted by trypan blue exclusion. PECs were adjusted to 5 × 106 cells/ml in RPMI supplemented and cultured in six-well plates (Costar, Cambridge, Mass.). After 3 h at 37°C and 5% CO2, nonadherent cells were removed by washing with warm supplemented RPMI medium. Adherent cells were removed with cold PBS and a scraper and readjusted to 106 cells/ml. Viability was determined at this step by trypan blue exclusion; viability was usually >95%. One milliliter was plated, and cell activation was performed in 24-well plates (Costar) by addition of lipopolysaccharide (LPS) (5 μg/ml, E. coli 111:B4; Sigma, St. Louis, Mo.) followed by incubation for 48 h at 37°C and 5% CO2. Supernatants were harvested, centrifuged, and examined by enzyme-linked immunosorbent assay (ELISA) for production of IL-6, IL-12, IL-10 (antibodies and cytokines were obtained from Pharmingen [San Diego, Calif.]), IL-18, and transforming growth factor beta (TGF-β) (obtained from R&D Systems). Peritoneal adherent cells constituted >90% of macrophages according to fluorescence-activated cell sorter (FACS) analysis (F4/80+).
Flow-cytometric analysis.
Expression of costimulatory and accessory molecules was analyzed by flow cytometry of peritoneal cells from uninfected mice and on weeks 2, 4, and 8 following T. crassiceps infection. PECs were obtained as mentioned above and cultured in 24-well plates (Costar) overnight at 37°C with 5% CO2. Nonadherent cells were discarded, and adherent cells were recovered with a plastic scraper and processed for analysis. Adherent PECs were blocked with anti-mouse FcγR antibody (CD16/CD32) and stained with fluorescein isothiocyanate-conjugated monoclonal antibodies against F4/80, MHC-II, B7-2, or B7-1 or phycoerythrin-conjugated antibodies against CD23 or CCR5. All antibodies were purchased from Pharmingen except F4/80, which was obtained from Serotec (Oxford, England). Stained cells were analyzed on a FACSCalibur analyzer using Cell Quest software (Becton Dickinson). Live cells were electronically gated using forward- and side-scatter parameters.
Detection of NO production.
NO production by macrophages was assayed by determining the increase in nitrite concentration (33) by the Griess reaction adapted to microwell plates (Costar). Briefly, 50 μl of culture supernatant was mixed with an equal volume of Griess reagent (1.5% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, 2.5% phosphoric acid) and incubated for 10 min at room temperature in the dark, and the absorbance was measured at 570 nm. Values were quantified using serial dilutions of sodium nitrite.
PGE2 measurement.
PGE2 concentrations in supernatants from macrophages stimulated with 5 μg of LPS/ml were measured by an enzymatic immunoassay kit (Cayman Chemical) following the producers' specifications.
Cell culture and coculture of macrophages-CD4+ cells: antigen presentation assay.
All cultures and cocultures were maintained in RPMI 1640 (Gibco BRL) supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin (Sigma)/ml, 5 × 10−5 M 2-β-mercaptoethanol (GIBCO BRL), and 10% fetal bovine serum (HyClone).
Spleen cells from T. crassiceps-infected mice were processed as described previously (38) and stimulated with 25 μg of soluble antigen of T. crassiceps/ml, supernatants were harvested 72 h after antigen stimulation, and IL-4, IL-13, and IFN-γ were evaluated by ELISA. Coculture of macrophages with primed CD4+ T cells was performed as follows: macrophages were obtained as described, adjusted to 106/ml, plated in 96-well plates (Costar), and maintained at 37°C with 5% CO2 for 1 h in the presence of keyhole limpets hemocyanin (KLH) (Sigma) or chicken egg albumin (OVA) (Sigma), 50 μg/ml. Splenocytes were prepared from mice previously i.p. injected (7 days earlier) with KLH (100 μg/mouse) or OVA (100 μg/mouse) without adjuvant and enriched for CD4+ T cells (>95% by FACS analysis) using CD4 magnetic cell sorter beads (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ T cells were plated in 96-well flat-bottom plates (Costar) which were precoated with macrophages from uninfected or infected mice at a 1:2 ratio (macrophages/CD4+) and loaded with OVA or KLH as mentioned above. Cultures were maintained at 37°C with 5% CO2 for 4 days, and then [3H]thymidine (activity, 185 GBq/mmol; Amersham, Aylesbury, England) (1 μCi/well) was added and incubated for a further 18 h. Cells were harvested on a 96-well harvester (Tomtec, Toku, Finland) and counted using a β-plate counter. Values are expressed as counts per minute from triplicate wells and are the result after subtracting counts per minute from cocultures in the absence of antigen. Supernatants from cocultures were harvested at 72 h and analyzed for IL-4, IL-6, IL-12, and IFN-γ production. In some cocultures (at 8 weeks after infection) PECs, previously loaded with OVA, were fixed with 0.5% paraformaldehyde for 5 to 10 min, washed extensively with RPMI, adjusted, and added to 96-well plates in the presence of CD4+ cells previously sensitized with OVA. These cocultures were processed as described above.
Statistical analysis.
Comparisons between control and experimental groups in this work were made using Student's unpaired t test. P values of <0.05 were considered significant.
RESULTS
Parasite growth and shifting from a Th1-type response towards a Th2-type response.
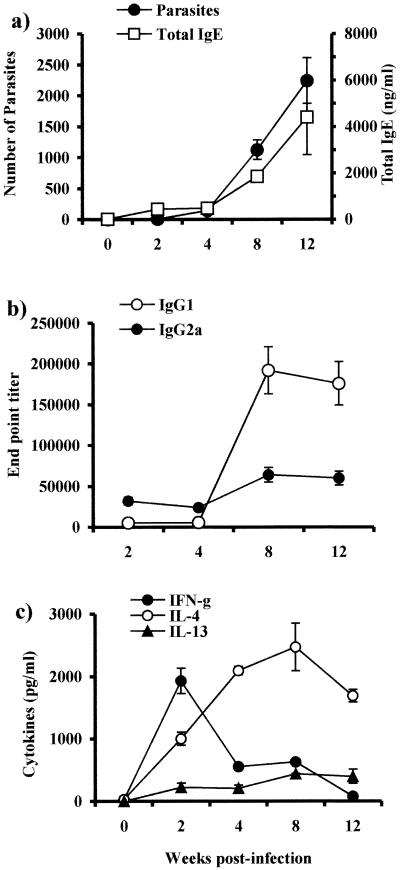
Studies in murine cysticercosis have shown that the immune response is altered by the chronicity of the infection, going from an early Th1-type to a late Th2-type response as infection progresses (41). Since we were interested in determining the mechanisms that induce highly polarized immune responses in helminth infections, we used this model to study how APCs derived from different immunological environments can alter or modify an expected immune response. In order to show that APCs used in our study were under the influence of a different immunological environment, we assayed the kinetics of parasite growth in the peritoneal cavity, the specific antibody response, and total immunoglobulin E (IgE) levels after an initial i.p. infection with 20 cysticerci. Furthermore, we analyzed the profile of cytokines released by splenocytes from infected mice after antigen-specific stimulation. The results obtained were similar to those previously described (38, 41), showing that at early infection IgG2a antigen-specific levels were higher than those detected for IgG1, but as T. crassiceps infection progressed, increased levels of both total IgE and antigen-specific IgG1 antibodies were detected while the IgG2a levels showed only a slight increase (Fig. 1a and b). On the other hand, antigen-specific IFN-γ was detected at higher levels early in the infection, but as infection turned chronic these levels dropped drastically. In contrast, IL-4 and IL-13 were detected in low levels early in the infection but were increased during chronic infection (Fig. 1c). These results show that although T. crassiceps-infected mice initially develop a Th1-type immune response concomitant with a limited parasite growth, later this immune response is progressively polarized towards a Th2-type response.
FIG. 1.
Course of T. crassiceps infection in BALB/c mice and Th1/Th2 markers. (a) Female BALB/c mice were inoculated i.p. with 20 cysticerci of T. crassiceps, and parasite loads and total IgE levels were monitored at times indicated. (b) Endpoint titers for antigen-specific antibody production of IgG1 and IgG2a. (c) Antigen-specific production of IFN-γ, IL-4, and IL-13 by splenocytes from T. crassiceps-infected mice as markers of Th1/Th2 environment. Data are representative of three independent experiments. Error bars represent ± standard deviations.
Helminth infection differentially alters macrophages' cytokine response in a time-dependent manner.
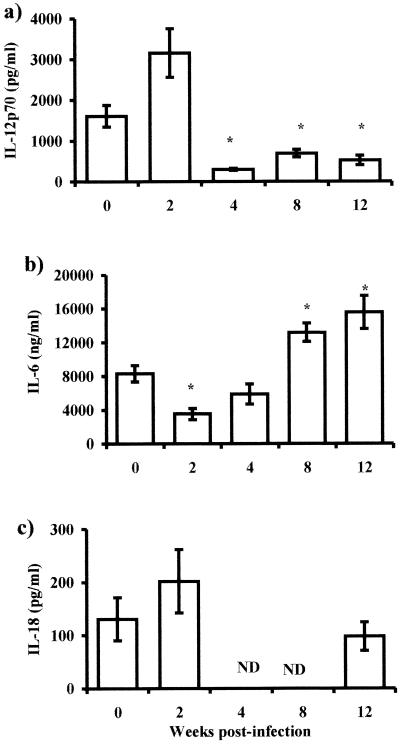
The mechanisms involved in the induction of polarized Th2-type responses in cysticercosis and in other helminth infections are still undefined. To determine the effect of T. crassiceps infection on macrophage function, we analyzed the cytokine production by adherent peritoneal exudate cells (PECs, mostly macrophages, >90% according to the expression of F4/80) from T. crassiceps-infected mice at weeks 2, 4, 8, and 12. Adherent macrophages were stimulated with LPS (5 μg/ml) for 48 h, and supernatants were analyzed for cytokine production (IL-6, IL-10, IL-12, IL-18, and TGF-β). Macrophages obtained in early periods after infection (2 weeks), when the parasite burden was low (Fig. 1a), produced levels of IL-12 similar to or higher than those for macrophages from uninfected mice (Fig. 2a). In contrast, IL-6 was detected in significantly lower levels in the same supernatants (Fig. 2b). Macrophages from late infections produced significantly higher levels of IL-6 (Fig. 2b, P < 0.05), but IL-12 was significantly suppressed between weeks 8 and 12 postinfection when the numbers of parasites were higher. At both time points there were no significant alterations in the levels of IL-18 (Fig. 2b). IL-10 and TGF-β levels were minimally detected in these same supernatants, and no statistically significant differences were observed in the infection (data not shown).
FIG. 2.
Cytokine production by macrophages in response to Th1-polarizing antigen (LPS). IL-12 (a), IL-6 (b), and IL-18 (c) production by macrophages from different weeks after T. crassiceps infection was determined by ELISA. Adherent PECs were obtained from infected mice at the times indicated, as well as from age-matched uninfected mice. Macrophages (106) were stimulated in vitro with 5 μg of LPS/ml for 48 h. Data are the mean ± standard deviation for six animals at each time point. Asterisk, P < 0.05 with respect to uninfected mice and mice at 2 weeks postinfection; ND, not determined.
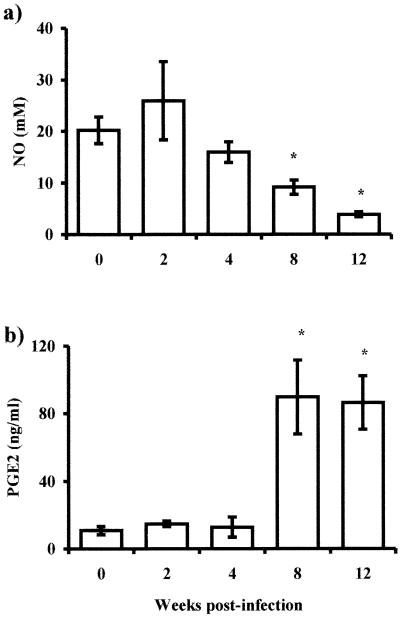
NO and PGE2 are two compounds associated with the state of macrophage activation (46). As shown in Fig. 3a, NO production was maintained in steady levels until the fourth week after infection; however, as the infection progressed (8 to 12 weeks), the NO levels dropped significantly (P < 0.05). In contrast to IL-12 and NO, PGE2 production presented a strikingly reverse pattern; that is, only low levels were released by adherent macrophages obtained early after infection, but as infection advanced, these cells produced significantly higher quantities of PGE2, reaching the maximum level at 8 weeks postinfection, which was sustained at least until week 12 postinfection (Fig. 3b, P < 0.01).
FIG. 3.
Other products of macrophages' activity are altered by T. crassiceps infection. Production of PGE2 (a) and NO (b) as measured by ELISA or Griess reaction were assayed from the same culture supernatants from Fig. 2. Asterisk, P < 0.05 with respect to uninfected mice and mice at 2 weeks postinfection.
Chronic helminth infection alters the APC function of adherent macrophages.
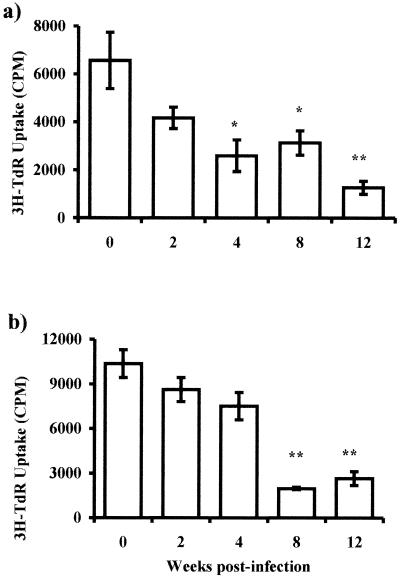
It is known that helminth infections can significantly bias immune responses to unrelated soluble antigens introduced in the host towards a Th2 type (8), but the mechanisms related to this phenomenon are not clear. Since adherent PECs are mostly macrophages and they can function as APC, we decided to investigate whether this property in our cell population could be affected by the course of the infection. To examine the APC ability of adherent PECs to prime Th responses in vitro, macrophages obtained at different times after infection or from healthy mice were cocultured with purified CD4+ T cells isolated from mice previously injected with KLH or OVA. Adherent macrophages were obtained and pulsed with antigen (KLH or OVA at 50 μg/ml), and after 2 h CD4+ T cells were plated in the same cultures. Adherent macrophages obtained from healthy or early-infected mice induced strong proliferative responses in CD4+ T cells to either KLH or OVA antigens (Fig. 4). In contrast, cocultures where the APC were obtained from chronically infected mice presented a significantly weaker ability to induce proliferative responses in CD4+ T cells (Fig. 4).
FIG. 4.
T. crassiceps infection alters the capacity of macrophages as antigen-presenting cells. Macrophages from different weeks after infection were loaded with KLH (a) or OVA (b) and cocultured with CD4+ cells (previously sensitized) for 5 days; proliferation was assayed by uptake of [3H]thymidine (3H-TdR). Data are representative of three different experiments, ± standard errors. Asterisk, P < 0.05; double asterisk, P < 0.01 (compared to uninfected mice or mice infected for 2 weeks).
The state of activation of APC alters the pattern of cytokines secreted by CD4+ T cells.
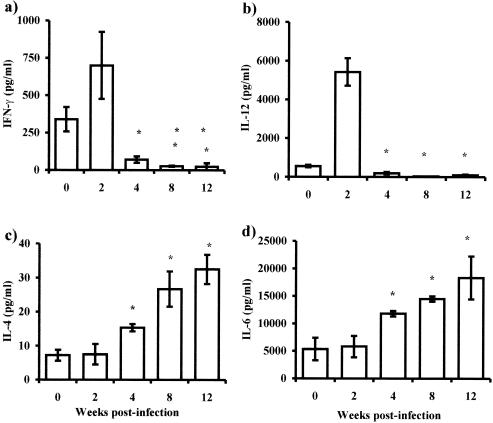
Parallel to the proliferative response, supernatants in the cocultures were harvested and analyzed for IL-4, IL-6, IL-12, and IFN-γ production. In supernatants from CD4+ T cells cocultured with APCs obtained in early infections, significantly higher levels of IFN-γ and IL-12 were detected than were detected with those stimulated with APCs from later infections (Fig. 5a and b). In addition, the same supernatants showed low levels of IL-4 and IL-6 in the CD4+ cells cocultured with adherent macrophages from uninfected or early-infected mice. However, as infection progressed, adherent macrophages drove CD4+ cells to produce significantly more IL-4 but lower levels of IFN-γ (Fig. 5c and a), whereas the levels of IL-6 in these supernatants were higher than in early infections (Fig. 5d).
FIG. 5.
T-cell-polarizing ability of alternatively activated macrophages in vivo during T. crassiceps infection. Adherent peritoneal macrophages were obtained from infected mice at the times indicated, as well as from age-matched uninfected mice. Cells (106) were loaded in vitro with 50 μg of OVA for 2 h. CD4+ T cells (2 × 106) from healthy mice previously immunized with OVA were cocultured, and 72 h later supernatants were analyzed for IFN-γ (a), IL-12 (b), IL-4 (c), and IL-6 (d). Data are representative of three independent experiments with at least four animals individually assayed. Error bars indicate ± standard deviations. Asterisk, P < 0.05 comparing with macrophages from healthy mice or early (2 weeks)-infected mice.
Differential expression of surface molecules is detected on macrophages, as infection turns chronic.
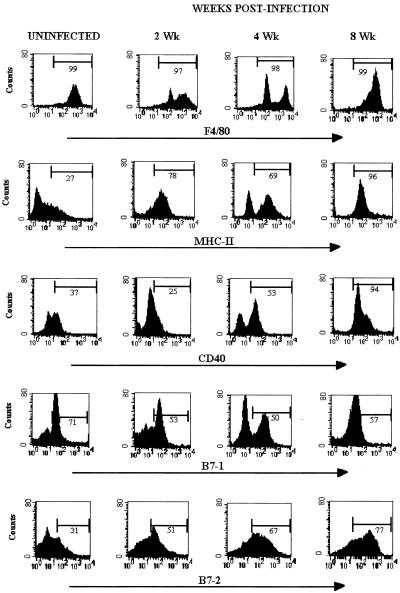
It has been shown that several molecules on the surface of the APC are important for adequate interaction with the responder T cells to induce them to proliferate and secrete cytokines. To determine whether infection with cysticerci could alter the antigen-presenting function in macrophages, the expression of costimulatory molecules and molecules related to antigen presentation were evaluated. We measured the expression of B7-1, B7-2, MHC-II, and CD40 on macrophages extracted from uninfected mice and from T. crassiceps-infected mice after 2, 4, and 8 weeks of infection. As infection progressed, macrophages showed an increase of the costimulatory molecules MHC-II and CD40. By 8 weeks postinfection, more of the 90% of macrophages expressed MHC-II and CD40 molecules with high density per cell (Fig. 6), suggesting that macrophages from chronic infection have increasing APC capacity. However, compared with uninfected mice there was not significant change in the surface expression of B7-1 at any point of the infection. In contrast, B7-2 expression was markedly up-regulated in macrophages from late-infected mice (8 weeks) with respect to its expression in uninfected mice and mice infected for 2 weeks (Fig. 6). The higher intensities of relative fluorescence observed in B7-2 expression indicate that chronic infection with T. crassiceps cysticerci can dramatically and differentially increase the expression of important molecules involved in antigen presentation.
FIG. 6.
Phenotypes of peritoneal macrophages isolated from T. crassiceps infection. Cell surface expression of F4/80, MHC-II, CD40, B7-1, and B7-2 at 0, 2, 4, and 8 weeks after T. crassiceps infection are shown. Data are representative histograms of three separate experiments.
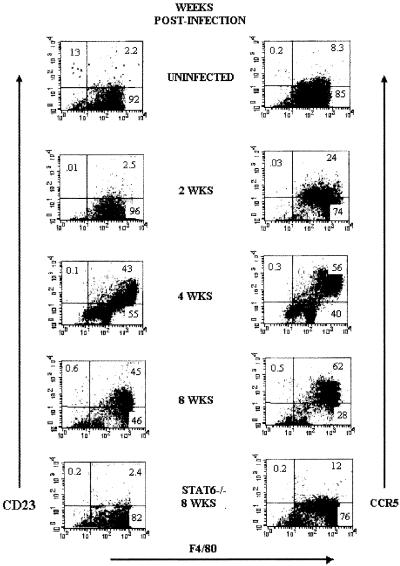
Because macrophages obtained from different times of helminth infection displayed both different patterns of cytokine production and distinct abilities to induce antigen-specific proliferation, we next looked for additional markers that identified these apparently different populations of macrophages. Double immunofluorescence analysis showed an interesting up-regulation of CD23 and CCR5 in F4/80+ cells (macrophages) as infection advanced (Fig. 7). Expression of CD23 has been associated with a state of activation of macrophages known as alternatively activated macrophages (11), whereas CCR5 expression has been basically associated with inflammatory processes (47). Interestingly, the up-regulation of these markers was accompanied by an increase in parasite load, poor IL-12 production, and a weak ability to stimulate CD4+ T cells.
FIG. 7.
T. crassiceps infection up-regulates the expression of CD23/CCR5 in macrophages. Cell surface expression of F4/80-CD23 and F4/80-CCR5 on peritoneal macrophages obtained at different weeks after infection. Bottom, macrophages obtained from STAT6-KO mice 8 weeks after T. crassiceps infection.
In an attempt to elucidate other factors that could influence the recruitment or activation of these alternatively activated macrophages, we analyzed the peritoneal population that was expanded in response to T. crassiceps infection in STAT6-KO mice. As shown in Fig. 7 (bottom panel), macrophages recruited in STAT6-KO mice after 8 weeks of infection did not up-regulate CD23 and/or CCR5, which were detected at levels very similar to those observed for uninfected mice. It is important to note that STAT6-KO mice are highly resistant to T. crassiceps infection and their macrophages produce higher levels of IL-12 (39).
Blockade of IL-6 and B7-2 and inhibition of PGE2 did not restore CD4 proliferation, but absence of STAT6 did.
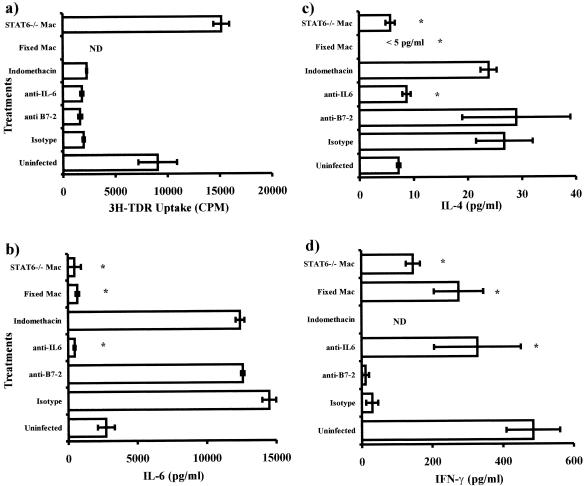
To determine factors contributing to the low level of proliferation observed in CD4+ T cells when macrophages from chronic infections were used as APC, we performed a series of experiments blocking IL-6, B7-2, and PGE2. As shown in Fig. 8, blockade of IL-6 and B7-2 with specific monoclonal antibodies and inhibition of PGE2 with indomethacin did not modify the poor ability to induce proliferation observed previously in macrophages from late infections (Fig. 8a). Interestingly, when macrophages obtained from STAT6-KO mice after 8 weeks of infection with T. crassiceps were used as APC, a significantly higher-level proliferative response of CD4+ T cells was observed (Fig. 8a). However, when the cytokine production was measured in the same cocultures, blockade of IL-6 significantly reduced the expected levels of IL-4, whereas IFN-γ was detected in significantly higher concentrations (Fig. 8c and d). Furthermore, we observed that blockade of B7-2 and PGE2 did not alter the cytokine profile previously detected (Fig. 8 b and d). In contrast, macrophages deficient in STAT6 signaling induced significantly lower levels of IL-4 but higher production of IFN-γ compared with wild-type macrophages (Fig. 8 c and d). Finally, in order to know the source of IL-6 detected in the cocultures, macrophages from chronic infections were loaded with OVA, fixed, and used as APC. Data from these cocultures showed that IL-6 is largely produced by macrophages, since these cocultures showed a very low level of IL-6 (Fig. 8b), and again levels of IFN-γ were recovered (IL-4 levels were <5 pg/ml) (Fig. 8 c and d). Thus, macrophages obtained from chronically T. crassiceps-infected mice potently drive Th2 development, apparently in an IL-6- and STAT6-dependent manner.
FIG. 8.
Effect of fixation, blockade of IL-6/B7-2, inhibition of PGE2, and absence of STAT6 signaling of macrophages in CD4+ T-cell proliferation and cytokine production. (a) Macrophages from mice at 8 weeks postinfection were used as APC and cocultured as for Fig. 4. In addition, blocking antibodies for IL-6 and B7-2, indomethacin, fixed macrophages, and STAT6-KO macrophages were used in these cocultures. (a) Proliferation was assayed by [3H]thymidine (3H-TDR) incorporation. (b to d) Cytokine levels detected in the presence and absence of blocking antibodies, indomethacin, fixed macrophages, or STAT6-KO macrophages. Asterisk, P < 0.05 compared to isotype control. ND, not determined.
DISCUSSION
Early and sustained interactions between the pathogen and the host participate in the establishment of an adaptive immune response against the pathogen. The majority of intracellular pathogens are potent stimulators of Th1 responses. For example, Mycobacterium, Listeria, Trypanosoma, and Toxoplasma all drive Th1-type responses, in part, due to the rapid induction of IL-12 and IFN-γ by innate immune cells responding through expressed molecules, termed pattern recognition receptors, which bind conserved structures shared by large groups of pathogens (3, 5). In contrast, helminth infections generally bias the immune response towards a Th2-type profile that may also be associated with induction of antigen-specific or nonspecific anergy (8, 38). The mechanisms involved in this phenomenon are not yet completely defined.
To date, cytokines and APC cells represent two of the major determinant factors in the differentiation of naïve and uncommitted CD4+ T cells into effectors Th1 and Th2 cells (17). In addition, the activation status of APC has been gaining importance as an explanation of how the final commitment of Th1/Th2 cells is achieved, since they are both a source of cytokines and a source of antigen (17). In the present study our data demonstrate that following chronic exposure to the helminth T. crassiceps, a population of macrophages with an increased expression of MHC-II, CD40, B7-2, CD23, and CCR5 is expanded. This population produces low levels of IL-12 both in response to LPS and in coculture with CD4+ T cells plus antigen and also exhibited a very limited ability to induce antigen-specific CD4+ T-cell proliferation. Moreover, CD4+ T cells stimulated with these APCs produced more IL-4 instead of IFN-γ. In contrast, macrophages obtained after a short period following the helminth infection (2 weeks) expressed low levels of B7-2, CD23, and CCR5, produced more IL-12, and induced high levels of proliferation in CD4+ T cells, which preferentially produced IFN-γ.
Recently it has been shown that exposure of immature APCs to helminth antigens in vitro can influence the Th1/Th2 commitment (18, 25, 26, 28, 30). However, this study is the first demonstration in vivo that over the course of a helminth infection APCs can undergo such a dramatic change in phenotype and be able to produce a differential pattern of cytokines in such a manner that on encountering a new antigen the immune response is biased towards Th2 differentiation.
lL-12 plays a critical role in the development of Th1 responses, up-regulating the production of IFN-γ by NK and CD4+ T cells (32). Our data showed that IL-12 production was down-regulated as infection progressed. In chronic infections, macrophages switch to a phenotype that produces more IL-6 and PGE2 but less IL-12 and NO. The absence of APC-derived IL-12 could be a default pathway in helminth infections to rapidly alter the outcome of immunity to new antigenic challenges or infections. In addition, the presence of IL-6 could facilitate this transition, since IL-6 has been shown to induce differentiation of IL-4-producing CD4+ T cells (37). Thus, IL-6 produced by APCs could play an important role in the initiation of Th2 differentiation in helminth infections, as has been recently suggested (23). In our study, this idea is supported by the findings that blockade of IL-6 in cocultured macrophages from late infections with CD4+ T cells suppressed IL-4 production. Furthermore, when macrophages were fixed and used as APC, levels of IL-6 were at minimum, and in both cases IFN-γ reached levels similar to those detected in cocultures with macrophages from healthy mice, suggesting that IL-6 may be an important factor favoring Th2 commitment in cysticercosis. Also, the higher level of production of PGE2 by macrophages from chronic infections could be associated with the low production of IL-12 and NO whereas favoring IL-6 production (12, 19, 22, 44). Thus, several APC-dependent factors are involved in the Th2 differentiation during a helminth infection.
Besides the cytokines produced by antigen-presenting cells, there are other factors, such as altered peptide ligands and costimulatory molecules (B7-1, B7-2), that can influence the differentiation of T cells (7, 24). We found that B7-2 expression was up-regulated as infection progressed, whereas B7-1 was not. Despite the fact that there is strong evidence of the involvement of B7-2 in Th2-biased responses in different systems (43), in helminth infections it has been shown that neither B7-1 nor B7-2 blockage affects the development of the immune response (9, 13). Similarly, in the present study blockage of B7-2 in vitro did not modify the outcome of IL-4/IFN-γ production or proliferation in cocultured CD4+ T cells/macrophages. These data suggest that costimulation through B7-2 is not involved in the induction of Th2-type responses by macrophages from late T. crassiceps infections.
Peritoneal macrophages taken from chronic infections displayed features shared with alternatively activated macrophages, which can be induced by IL-4, IL-13, IL-10, and TGF-β, and they express innate immune receptors and CD23 (10, 11). These alternatively activated macrophages have been reported in filariasis, where they appear to play a role in inducing Th2 differentiation (25, 29). Moreover, these macrophages also produce IL-10 and TGF-β and apparently play an important role in down-modulating inflammation and immunity. Though our results differed in IL-10 and TGF-β involvement and in the different surface markers, our data on IL-4 dependence, low-level induction of proliferative ability, time required to appear, and Th2 biasing activity of alternatively activated macrophages in murine cysticercosis are in accordance with the findings of MacDonald et al. (28) and Loke et al. (26), who implanted the nematode Brugia malayi in the peritoneal cavity of mice to recruit suppressor macrophages. Loke et al. also showed that cytokine production in their system was skewed towards the Th2 type, paralleled with suppression in the proliferative response (25). Together, these studies suggest that the commitment towards a Th2 pattern of cytokine production by CD4+ cells following interaction with alternatively activated macrophages may explain the reduced levels of IFN-γ and IL-12 observed with certain helminth infections and the biased Th2-type response to new antigenic challenges consistently reported in helminth coinfections (1, 21, 22).
In our model, macrophages isolated from a chronic infection shared some of the features observed in alternatively activated macrophages, as mentioned before, and in addition they expressed high levels of CCR5/CD23 as well as molecules associated with antigen presentation. Interestingly, peritoneal macrophages taken from STAT6−/− T. crassiceps-infected mice (8 weeks) did not show these features. They had low CD23 and CCR5 expression and successfully induced antigen-specific proliferation as well as IFN-γ production in CD4+ T cells when they were used as APC, revealing that the development of these alternatively activated macrophages is critically dependent on the STAT6-mediated signaling pathway and also implying a role for IL-4 and/or IL-13 in the expansion and activation of these macrophages during this helminth infection.
An exciting question regards the early source of IL-4 in response to helminth antigens; accessory cells and naïve Th cells have been suggested as sources of this key cytokine (14, 27), but conclusive studies are still missing. In our hands, ex vivo analyses of macrophages from T. crassiceps-infected mice (with no further in vitro stimulation) by reverse transcription-PCR suggest that these cells are not a source of IL-4 (data not shown). However, further studies are necessary to know whether or not helminth antigen does stimulate macrophages directly to produce IL-4; this is an area of growing interest (27).
The role of high-level expression of CCR5 in macrophages is not well understood (20); however, in T cells it has been associated with inflammation and is IL-12 dependent (35). The high-level expression of CCR5 detected in macrophages from heavily parasitized mice could be related to the absence of CCR5 ligands, RANTES/MIP-1α (31). On the other hand, the relationship between CCR5 in macrophages and their poor ability to induce proliferation in CD4+ T cells is an interesting finding that needs more detailed study. Indeed, it is noteworthy that this population of macrophages produces low levels of IL-12 even in response to a strong Th1-type stimuli, such as LPS (30), and also in the cocultures with antigen-specific CD4+ T cells where the CD40-CD40L system is participating. These observations are in sharp contrast with dendritic cells expressing high levels of CCR5 in response to acute exposure to protozoan antigens, which were high producers of IL-12 and play a significant role in inducing Th1 responses (3, 32). This discrepancy between failure to produce IL-12 and high CCR5 expression in macrophages from chronic T. crassiceps-infected mice could be an intrinsic feature for helminth infections and possibly plays a role in the bias towards Th2 immune responses to both the parasite's antigen and nonrelated antigens in helminthic diseases (1, 15, 38).
In conclusion, we found that chronic infection with the helminth parasite T. crassiceps can differentially regulate the T-cell-stimulatory ability of APC and favor a Th2-type response to new antigenic encounters. This mechanism appears to be STAT6 dependent and possibly involve IL-4 and IL-13. However, a recent body of evidence suggests an alternative route to Th2 biasing in helminth infections, which implicates APCs as a possible direct target of helminth products to finally deliver Th2 signals (30, 40, 42).
Acknowledgments
This work was partially supported by CONACYT-Mexico, grant number 31102-M. M. Rodriguez-Sosa and L. I. Terrazas hold a CONACYT-fellowship.
We thank Maureen Drakes for her critical review and suggestions.
Editor: J. M. Mansfield
REFERENCES
- 1.Actor, J. K., M. Shirai, M. C. Kullberg, R. M. Buller, A. Sher, and J. A. Berzofsky. 1993. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA 90:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso, L. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. [DOI] [PubMed] [Google Scholar]
- 3.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat. Immunol. 1:83-87. [DOI] [PubMed] [Google Scholar]
- 4.Allen, J. E., and P. Loke. 2001. Divergent roles for macrophages in lymphatic filariasis. Parasite Immunol. 23:345-352. [DOI] [PubMed] [Google Scholar]
- 5.Bliss, S. K., B. A. Butcher, and E. Y. Denkers. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515-4521. [DOI] [PubMed] [Google Scholar]
- 6.Bottomly, K. 1999. T cells and dendritic cells get intimate. Science 283:1124-1125. [DOI] [PubMed] [Google Scholar]
- 7.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 8.Cox, F. E. 2001. Concomitant infections, parasites and immune responses. Parasitology 122(Suppl.):S23-S38. [DOI] [PubMed] [Google Scholar]
- 9.Gause, W. C., P. Lu, X. D. Zhou, S. J. Chen, K. B. Madden, S. C. Morris, P. S. Linsley, F. D. Finkelman, and J. F. Urban. 1996. H. polygyrus: B7-independence of the secondary type 2 response. Exp. Parasitol. 84:264-273. [DOI] [PubMed] [Google Scholar]
- 10.Goerdt, S., and C. E. Orfanos. 1999. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10:137-142. [DOI] [PubMed] [Google Scholar]
- 11.Goerdt, S., O. Politz, K. Schledzewski, R. Birk, A. Gratchev, P. Guillot, N. Hakiy, C. D. Klemke, E. Dippel, V. Kodelja, and C. E. Orfanos. 1999. Alternative versus classical activation of macrophages. Pathobiology 67:222-226. [DOI] [PubMed] [Google Scholar]
- 12.Gomi, K., F. G. Zhu, and J. S. Marshall. 2000. Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J. Immunol. 165:6545-6552. [DOI] [PubMed] [Google Scholar]
- 13.Greenwald, R. J., P. Lu, M. J. Halvorson, X. Zhou, S. Chen, K. B. Madden, P. J. Perrin, S. C. Morris, F. D. Finkelman, R. Peach, P. S. Linsley, J. F. Urban, Jr., and W. C. Gause. 1997. Effects of blocking B7-1 and B7-2 interactions during a type 2 in vivo immune response. J. Immunol. 158:4088-4096. [PubMed] [Google Scholar]
- 14.Hayashi, N., K. Matsui, H. Tsutsui, Y. Osada, R. T. Mohamed, H. Nakano, S. Kashiwamura, Y. Hyodo, K. Takeda, S. Akira, T. Hada, K. Higashino, S. Kojima, and K. Nakanishi. 1999. Kupffer cells from Schistosoma mansoni-infected mice participate in the prompt type 2 differentiation of hepatic T cells in response to worm antigens. J. Immunol. 163:6702-6711. [PubMed] [Google Scholar]
- 15.Helmby, H., M. Kullberg, and M. Troye-Blomberg. 1998. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect. Immun. 66:5167-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland, M. J., Y. M. Harcus, P. L. Riches, and R. M. Maizels. 2000. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 30:1977-1987. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic, D., Z. Liu, and W. C. Gause. 2001. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22:450-457. [DOI] [PubMed] [Google Scholar]
- 18.Jenne, L., J. F. Arrighi, B. Sauter, and P. Kern. 2001. Dendritic cells pulsed with unfractionated helminthic proteins to generate antiparasitic cytotoxic T lymphocyte. Parasite Immunol. 23:195-201. [DOI] [PubMed] [Google Scholar]
- 19.Kalinski, P., C. M. Hilkens, A. Snijders, F. G. Snijdewint, and M. L. Kapsenberg. 1997. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 159:28-35. [PubMed] [Google Scholar]
- 20.Kaufmann, A., R. Salentin, D. Gemsa, and H. Sprenger. 2001. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J. Leukoc. Biol. 69:248-252. [PubMed] [Google Scholar]
- 21.Kullberg, M. C., E. J. Pearce, S. E. Hieny, A. Sher, and J. A. Berzofsky. 1992. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J. Immunol. 148:3264-3270. [PubMed] [Google Scholar]
- 22.Kuroda, E., Y. Yoshida, B. En Shan, and U. Yamashita. 2001. Suppression of macrophage interleukin-12 and tumour necrosis factor-alpha production in mice infected with Toxocara canis. Parasite Immunol. 23:305-311. [DOI] [PubMed] [Google Scholar]
- 23.La Flamme, A. C., A. S. MacDonald, and E. J. Pearce. 2000. Role of IL-6 in directing the initial immune response to schistosome eggs. J. Immunol. 164:2419-2426. [DOI] [PubMed] [Google Scholar]
- 24.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 25.Loke, P., A. S. MacDonald, and J. E. Allen. 2000. Antigen-presenting cells recruited by Brugia malayi induce Th2 differentiation of naive CD4(+) T cells. Eur. J. Immunol. 30:1127-1135. [DOI] [PubMed] [Google Scholar]
- 26.Loke, P., A. S. MacDonald, A. Robb, R. M. Maizels, and J. E. Allen. 2000. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur. J. Immunol. 30:2669-2678. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald, A. S., M. I. Araujo, and E. J. Pearce. 2002. Immunology of parasitic helminth infections. Infect. Immun. 70:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald, A. S., P. Loke, and J. E. Allen. 1999. Suppressive antigen-presenting cells in Helminth infection. Pathobiology 67:265-268. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald, A. S., R. M. Maizels, R. A. Lawrence, I. Dransfield, and J. E. Allen. 1998. Requirement for in vivo production of IL-4, but not IL-10, in the induction of proliferative suppression by filarial parasites. J. Immunol. 160:4124-4132. [PubMed] [Google Scholar]
- 30.MacDonald, A. S., A. D. Straw, B. Bauman, and E. J. Pearce. 2001. CD8(-) dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 167:1982-1988. [DOI] [PubMed] [Google Scholar]
- 31.Mack, M., J. Cihak, C. Simonis, B. Luckow, A. E. Proudfoot, J. Plachy, H. Bruhl, M. Frink, H. J. Anders, V. Vielhauer, J. Pfirstinger, M. Stangassinger, and D. Schlondorff. 2001. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166:4697-4704. [DOI] [PubMed] [Google Scholar]
- 32.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 33.Migliorini, P., G. Corradin, and S. B. Corradin. 1991. Macrophage NO2-production as a sensitive and rapid assay for the quantitation of murine IFN-gamma. J. Immunol. Methods 139:107-114. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 35.Mukai, T., M. Iwasaki, P. Gao, M. Tomura, Y. Yashiro-Ohtani, S. Ono, M. Murai, K. Matsushima, M. Kurimoto, M. Kogo, T. Matsuya, H. Fujiwara, and T. Hamaoka. 2001. IL-12 plays a pivotal role in LFA-1-mediated T cell adhesiveness by up-regulation of CCR5 expression. J. Leukoc. Biol. 70:422-430. [PubMed] [Google Scholar]
- 36.Okano, M., A. R. Satoskar, K. Nishizaki, M. Abe, and D. A. Harn, Jr. 1999. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 163:6712-6717. [PubMed] [Google Scholar]
- 37.Rincon, M., J. Anguita, T. Nakamura, E. Fikrig, and R. A. Flavell. 1997. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez, M., L. I. Terrazas, R. Marquez, and R. Bojalil. 1999. Susceptibility to Trypanosoma cruzi is modified by a previous non-related infection. Parasite Immunol. 21:177-185. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Sosa, M., J. R. David, R. Bojalil, A. R. Satoskar, and L. I. Terrazas. 2002. Cutting edge: susceptibility to the larval stage of the helminth parasite Taenia crassiceps is mediated by Th2 response induced via STAT-6 signaling. J. Immunol. 168:3135-3139. [DOI] [PubMed]
- 40.Semnani, R. T., H. Sabzevari, R. Iyer, and T. B. Nutman. 2001. Filarial antigens impair the function of human dendritic cells during differentiation. Infect. Immun. 69:5813-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terrazas, L. I., R. Bojalil, T. Govezensky, and C. Larralde. 1998. Shift from an early protective Th1-type immune response to a late permissive Th2-type response in murine cysticercosis (Taenia crassiceps). J. Parasitol. 84:74-81. [PubMed] [Google Scholar]
- 42.Terrazas, L. I., K. L. Walsh, D. Piskorska, E. McGuire, and D. A. Harn, Jr. 2001. The schistosome oligosaccharide lacto-N-neotetraose expands gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. J. Immunol. 167:5294-5303. [DOI] [PubMed] [Google Scholar]
- 43.Tsuyuki, S., J. Tsuyuki, K. Einsle, M. Kopf, and A. J. Coyle. 1997. Costimulation through B7-2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J. Exp. Med. 185:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Pouw Kraan, T. C., L. C. Boeije, A. Snijders, R. J. Smeenk, J. Wijdenes, and L. A. Aarden. 1996. Regulation of IL-12 production by human monocytes and the influence of prostaglandin E2. Ann. N. Y. Acad. Sci. 795:147-157. [DOI] [PubMed] [Google Scholar]
- 45.Villa, O. F., and R. E. Kuhn. 1996. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology 112:561-570. [DOI] [PubMed] [Google Scholar]
- 46.Williams, J. A., C. H. Pontzer, and E. Shacter. 2000. Regulation of macrophage interleukin-6 (IL-6) and IL-10 expression by prostaglandin E2: the role of p38 mitogen-activated protein kinase. J. Interferon Cytokine Res. 20:291-298. [DOI] [PubMed] [Google Scholar]
- 47.Yang, Y. F., M. Tomura, M. Iwasaki, T. Mukai, P. Gao, S. Ono, J. P. Zou, G. M. Shearer, H. Fujiwara, and T. Hamaoka. 2001. IL-12 as well as IL-2 upregulates CCR5 expression on T cell receptor-triggered human CD4+ and CD8+ T cells. J. Clin. Immunol. 21:116-125. [DOI] [PubMed] [Google Scholar]