Abstract
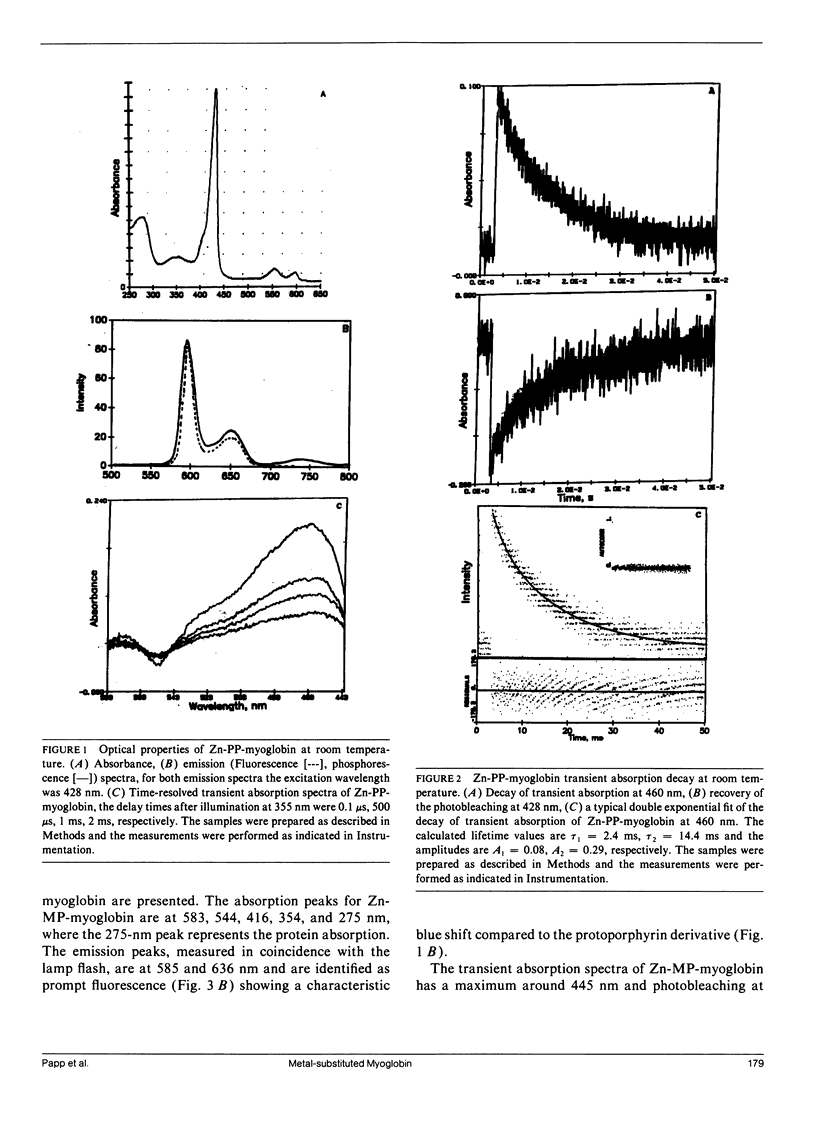
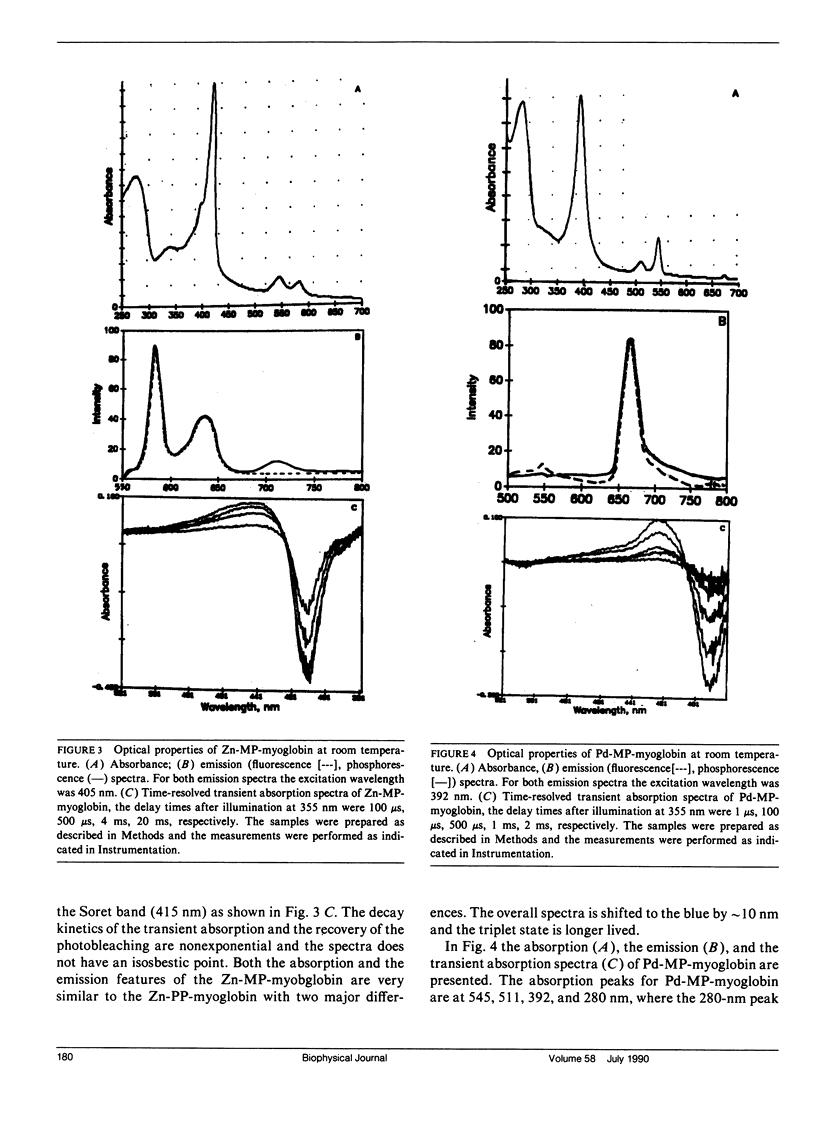
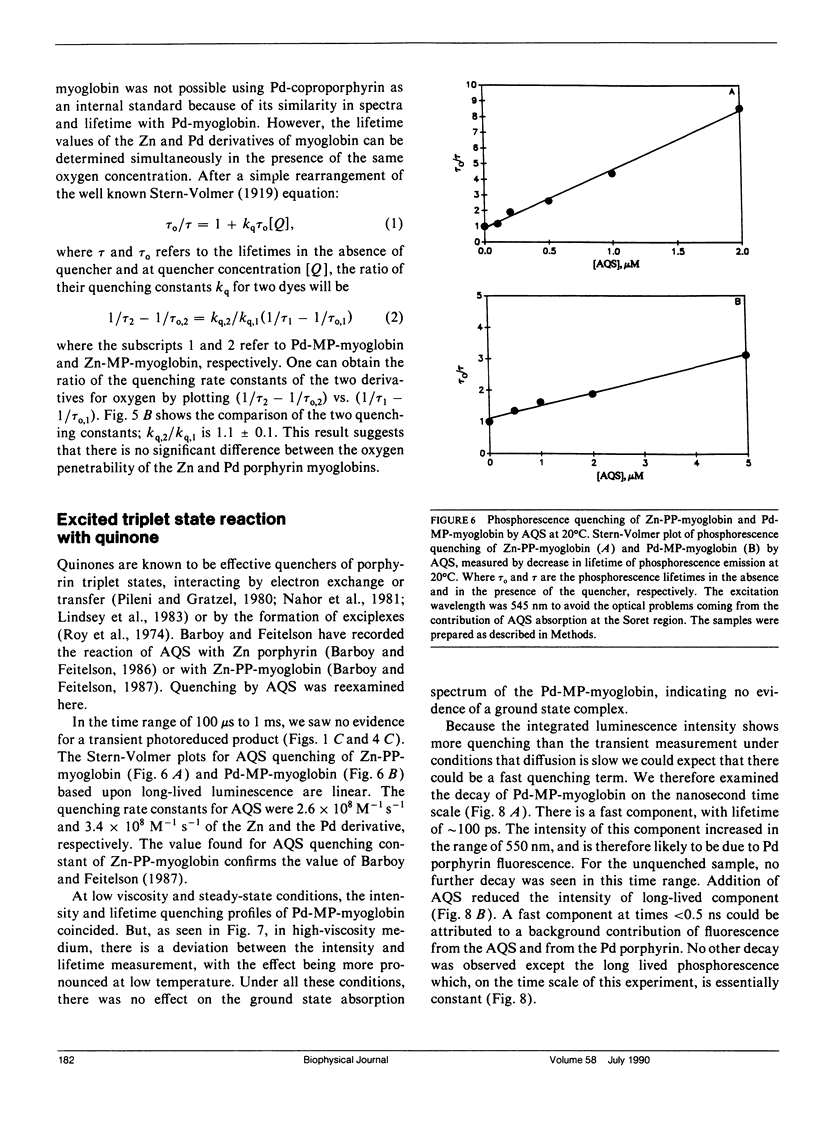
The triplet state absorption and phosphorescence of Zn and Pd derivatives of myoglobin were compared. Both metal derivatives exhibit long triplet state lifetimes at room temperature, but whereas the Pd derivative showed exponential decay and an isosbestic point in the transient absorption spectra, the decay of the Zn derivative was nonsingle exponential and the transient absorption spectra showed evidence of more than one excited state species. No difference was seen in triplet quenching by oxygen for either derivative, indicating that differences in the polypeptide chain between the two derivatives are not large enough to affect oxygen penetrability. Quenching was also observed by anthraquinone sulfonate. In this case, the possibility of long-range transfer by an exchange mechanism is considered.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albani J., Alpert B. Fluctuation domains in myoglobin. Fluorescence quenching studies. Eur J Biochem. 1987 Jan 2;162(1):175–178. doi: 10.1111/j.1432-1033.1987.tb10558.x. [DOI] [PubMed] [Google Scholar]
- Alpert B., Lindqvist L. Porphyrin triplet state probing the diffusion of oxygen in hemoglobin. Science. 1975 Mar 7;187(4179):836–838. doi: 10.1126/science.1114327. [DOI] [PubMed] [Google Scholar]
- Andres S. F., Atassi M. Z. Conformational studies on modified proteins and peptides. Artificial myoglobins prepared with modified and metalloporphyrins. Biochemistry. 1970 May 26;9(11):2268–2275. doi: 10.1021/bi00813a007. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Chan S. S. The rate of entry of dioxygen and carbon monoxide into myoglobin. Biophys J. 1978 Oct;24(1):175–186. doi: 10.1016/S0006-3495(78)85354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboy N., Feitelson J. Diffusion of small molecules through the structure of myoglobin. Environmental effects. Biochemistry. 1989 Jun 27;28(13):5450–5456. doi: 10.1021/bi00439a020. [DOI] [PubMed] [Google Scholar]
- Barboy N., Feitelson J. Quenching of the zinc-protoporphyrin triplet state as a measure of small-molecule diffusion through the structure of myoglobin. Biochemistry. 1987 Jun 2;26(11):3240–3244. doi: 10.1021/bi00385a046. [DOI] [PubMed] [Google Scholar]
- Calhoun D. B., Englander S. W., Wright W. W., Vanderkooi J. M. Quenching of room temperature protein phosphorescence by added small molecules. Biochemistry. 1988 Nov 1;27(22):8466–8474. doi: 10.1021/bi00422a026. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Ghiron C., Bazin M., Santus R. Determination of the dioxygen quenching constant for protein and model indole triplets. Biochim Biophys Acta. 1988 Nov 23;957(2):207–216. doi: 10.1016/0167-4838(88)90274-9. [DOI] [PubMed] [Google Scholar]
- Jameson D. M., Gratton E., Weber G., Alpert B. Oxygen distribution and migration within Mbdes Fe and Hbdes Fe. Multifrequency phase and modulation fluorometry study. Biophys J. 1984 Apr;45(4):795–803. doi: 10.1016/S0006-3495(84)84224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes S. M., Dalickas G. A., Eaton W. A., Hochstrasser R. M. Picosecond transient absorption study of photodissociated carboxy hemoglobin and myoglobin. Biophys J. 1988 Sep;54(3):545–549. doi: 10.1016/S0006-3495(88)82987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloczek H., Horie T., Yonetani T., Anni H., Maniara G., Vanderkooi J. M. Interaction between cytochrome c and cytochrome c peroxidase: excited-state reactions of zinc- and tin-substituted derivatives. Biochemistry. 1987 Jun 2;26(11):3142–3148. doi: 10.1021/bi00385a030. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M., Calhoun D. B., Englander S. W. On the prevalence of room-temperature protein phosphorescence. Science. 1987 May 1;236(4801):568–569. doi: 10.1126/science.3576185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J. M., Maniara G., Green T. J., Wilson D. F. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987 Apr 25;262(12):5476–5482. [PubMed] [Google Scholar]



