Abstract
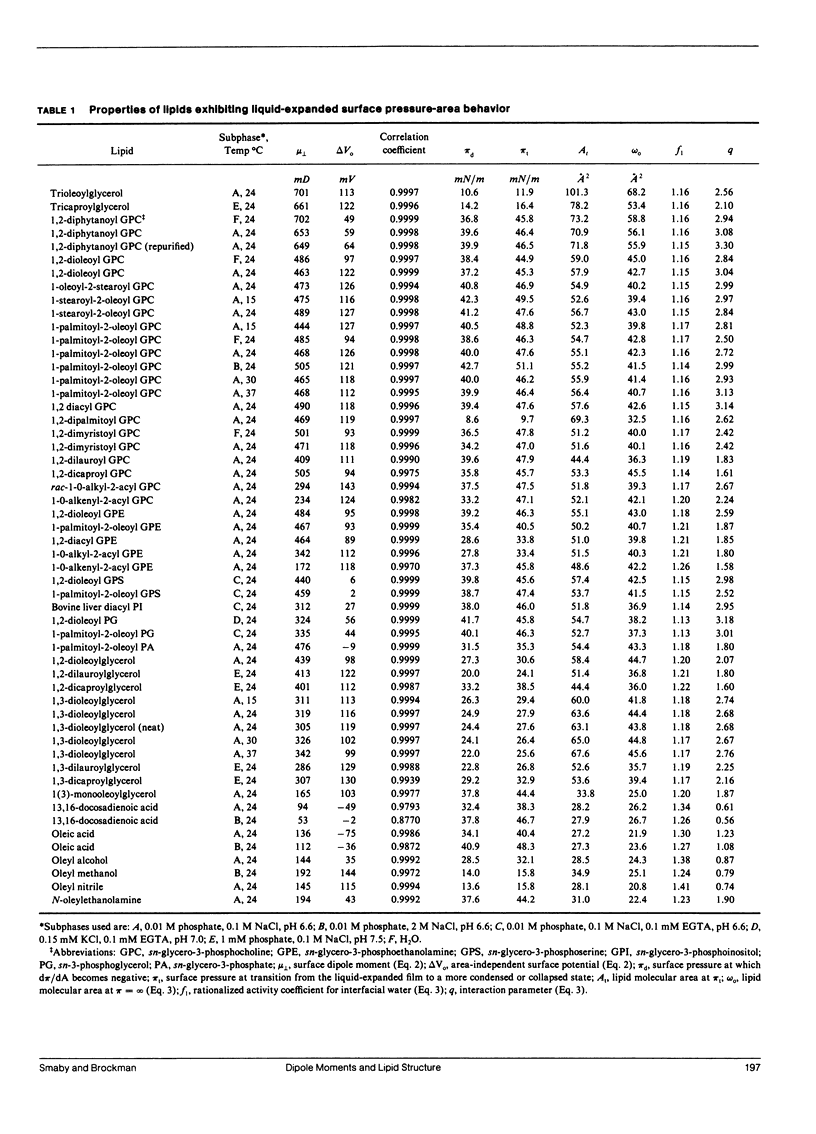
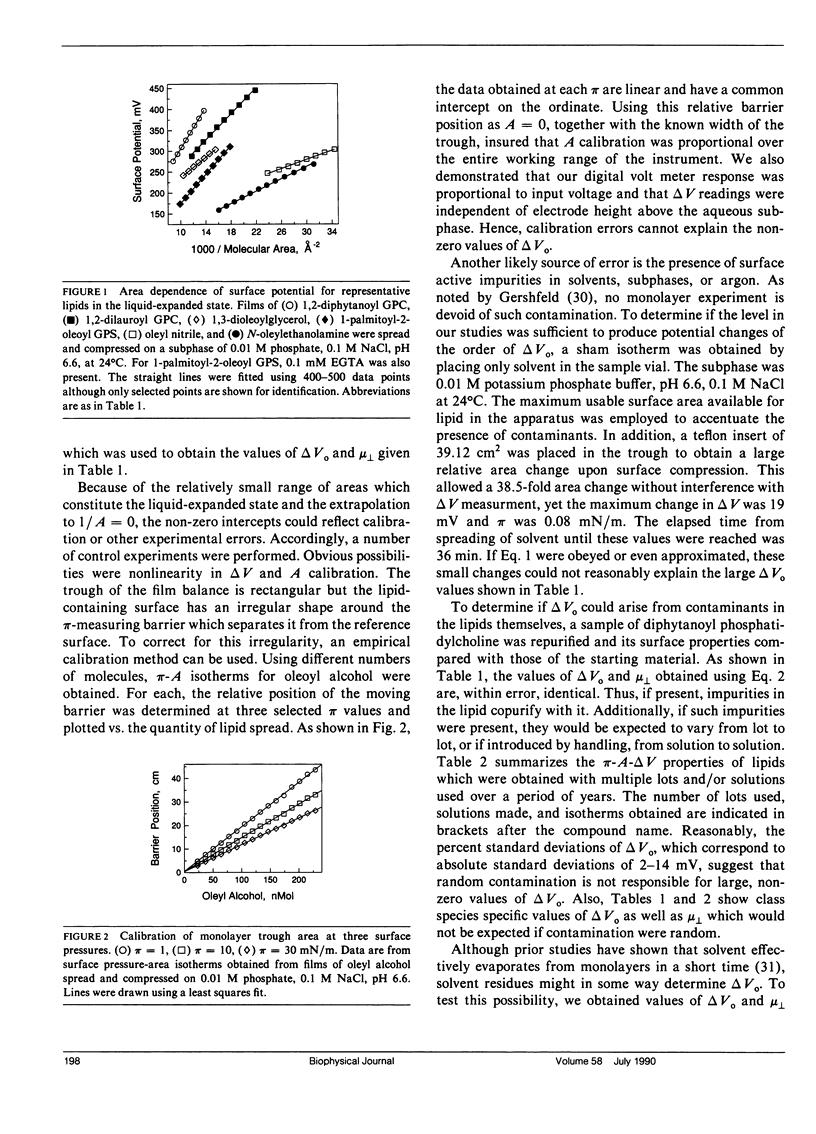
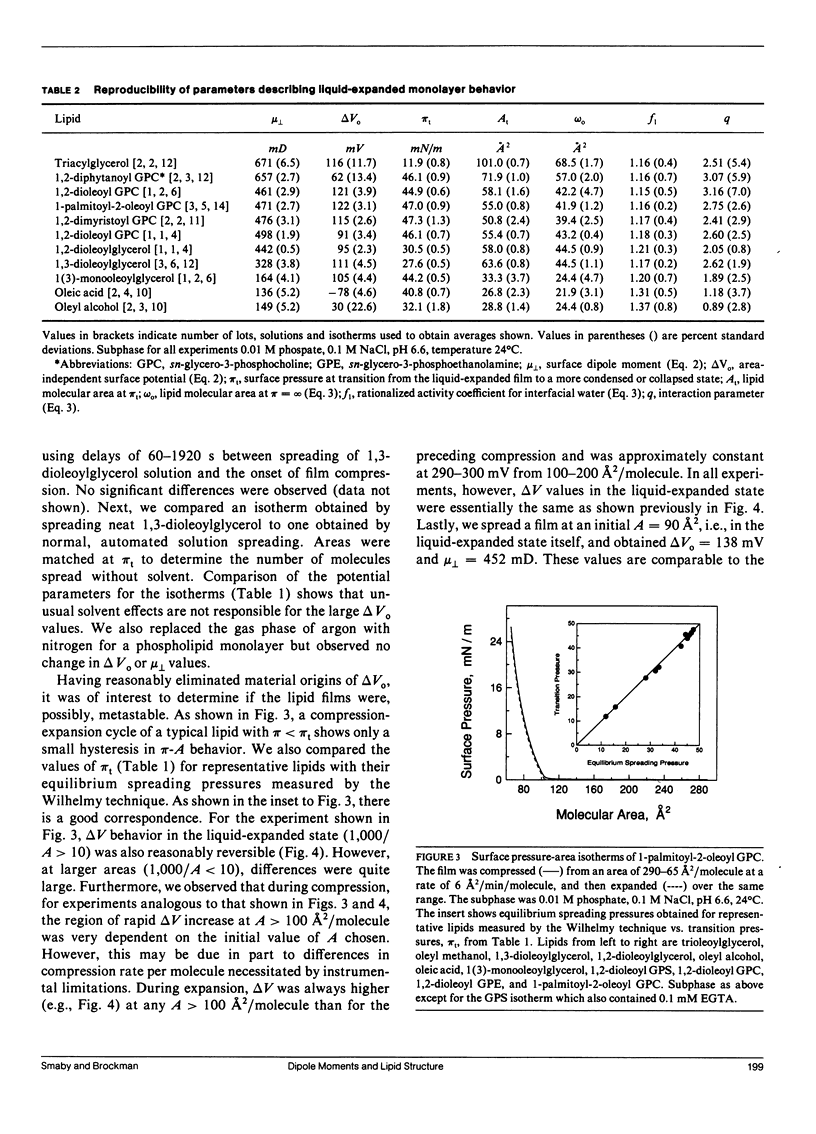
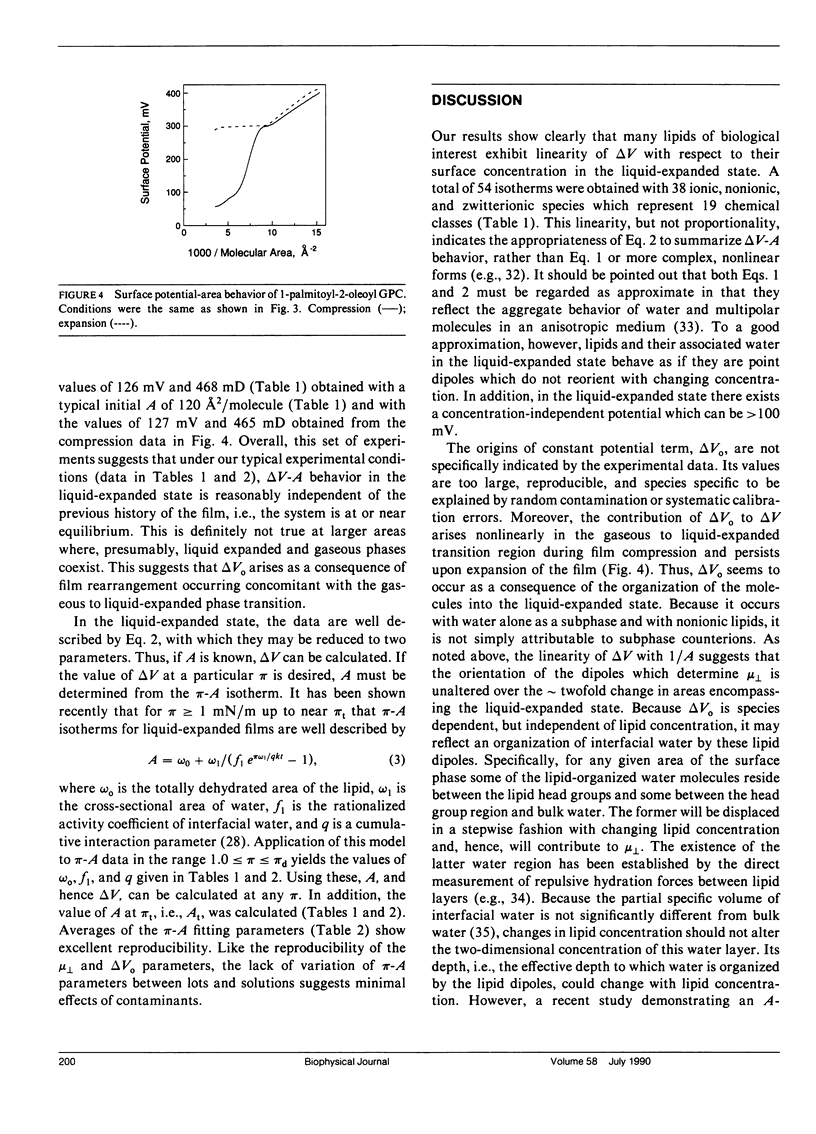
Surface potential-surface pressure-area isotherms at the argon-buffer interface have been determined for 38 lipid species comprising 19 chemical classes. These lipids all exhibited a finite range of liquid-expanded surface pressure-area behavior. For most species, the linearity of surface potential with reciprocal area was excellent, but nonzero intercepts were obtained. This suggests a lipid-induced reorganization of interfacial water molecules which is area independent. The linearity of the data permits calculation of the surface dipole moment, mu perpendicular, for each lipid. The values of mu perpendicular for a series of oleoyl-containing acylglycerols, dioleoyl phosphatidylcholine, and dioleoyl phosphatidylethanolamine exhibit acylglycerol ester group mu perpendicular's which are generally consistent with known conformational properties of such lipids. The values are 132 mD for the perpendicular oleoyl glycerol-ester group and 252 mD for that in the kinked-chain conformation. Comparison of mu perpendicular's calculated using these values with homologues confirms the approximate independence of mu perpendicular from aliphatic chain length and permits identification of exceptions with possible conformational or orientational differences. Notably, diphytanoyl phosphatidylcholine shows a 45% larger mu perpendicular than predicted. Differences in mu perpendicular among lipid classes allow estimation of the electrical consequences of lipid metabolism and exchange. Calculations show that reactions such as the generation of 1,2-diacylglycerol from diacyl glycerophosphocholine or diacyl glycerophosphoinositol should produce surface potential changes of -127 and +42 mV, respectively. Thus, the two phospholipids are not simply alternative sources of diacylglycerol with respect to processes dependent on surface potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agwu D. E., McPhail L. C., Chabot M. C., Daniel L. W., Wykle R. L., McCall C. E. Choline-linked phosphoglycerides. A source of phosphatidic acid and diglycerides in stimulated neutrophils. J Biol Chem. 1989 Jan 25;264(3):1405–1413. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beitinger H., Vogel V., Möbius D., Rahmann H. Surface potentials and electric dipole moments of ganglioside and phospholipid bilayers: contribution of the polar headgroup at the water/lipid interface. Biochim Biophys Acta. 1989 Sep 18;984(3):293–300. doi: 10.1016/0005-2736(89)90296-4. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Tsujita T., Brockman H. L. Enzymatic and physical characterization of diacylglycerol-phosphatidylcholine interactions in bilayers and monolayers. Biochemistry. 1989 Jan 10;28(1):32–40. doi: 10.1021/bi00427a006. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Role of phosphoinositides in the regulation of liver function. Hepatology. 1988 Jan-Feb;8(1):152–166. doi: 10.1002/hep.1840080129. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Fernández J. C., Aranda F. J., Micol V., Villalaín J., Ortiz A. Effect of diacylglycerols on calcium-induced fusion of phosphatidylserine/phosphatidylcholine vesicles. Biochem Soc Trans. 1989 Dec;17(6):957–960. doi: 10.1042/bst0170957. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Small D. M. Solubilization and localization of triolein in phosphatidylcholine bilayers: a 13C NMR study. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6878–6882. doi: 10.1073/pnas.78.11.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Elliott J. R. Surface potential changes in lipid monolayers and the 'cut-off' in anaesthetic effects of N-alkanols. Biochim Biophys Acta. 1986 Dec 16;863(2):337–340. doi: 10.1016/0005-2736(86)90278-6. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A. Lipid monolayer states and their relationships to bilayers. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4089–4093. doi: 10.1073/pnas.84.12.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Wiener M. C. Structure of fully hydrated bilayer dispersions. Biochim Biophys Acta. 1988 Jul 7;942(1):1–10. doi: 10.1016/0005-2736(88)90268-4. [DOI] [PubMed] [Google Scholar]
- Oki S. Letter: Evidence for a new concept of membrane potential. J Theor Biol. 1973 Dec;42(3):593–596. doi: 10.1016/0022-5193(73)90250-6. [DOI] [PubMed] [Google Scholar]
- Paltauf F., Hauser H., Phillips M. C. Monolayer characteristics of some 1,2-diacyl, I-alkyl-2-acyl and 1,2-dialkyl phospholipids at the air-water interface. Biochim Biophys Acta. 1971 Dec 3;249(2):539–547. doi: 10.1016/0005-2736(71)90129-5. [DOI] [PubMed] [Google Scholar]
- Pascher I., Sundell S., Hauser H. Glycerol conformation and molecular packing of membrane lipids. The crystal structure of 2,3-dilauroyl-D-glycerol. J Mol Biol. 1981 Dec 15;153(3):791–806. doi: 10.1016/0022-2836(81)90419-8. [DOI] [PubMed] [Google Scholar]
- Pownall H. J., Pao Q., Brockman H. L., Massey J. B. Inhibition of lecithin-cholesterol acyltransferase by diphytanoyl phosphatidylcholine. J Biol Chem. 1987 Jul 5;262(19):9033–9036. [PubMed] [Google Scholar]
- Seelig J., Macdonald P. M., Scherer P. G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987 Dec 1;26(24):7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Magnitude of the solvation pressure depends on dipole potential. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9263–9267. doi: 10.1073/pnas.86.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Miscibility, chain packing, and hydration of 1-palmitoyl-2-oleoyl phosphatidylcholine and other lipids in surface phases. Biophys J. 1985 Nov;48(5):701–707. doi: 10.1016/S0006-3495(85)83828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Novel surface phase containing cholesteryl esters. 1. Structural characteristics determined from surface pressure--area measurements. Biochemistry. 1981 Feb 17;20(4):718–723. doi: 10.1021/bi00507a008. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Regulation of cholesteryl oleate and triolein miscibility in monolayers and bilayers. J Biol Chem. 1987 Jun 15;262(17):8206–8212. [PubMed] [Google Scholar]
- Tatulian S. A. Fluidity-dependence of membrane adhesiveness can be explained by thermotropic shifts in surface potential. Biochim Biophys Acta. 1987 Jul 10;901(1):161–165. doi: 10.1016/0005-2736(87)90268-9. [DOI] [PubMed] [Google Scholar]
- Thuren T., Tulkki A. P., Virtanen J. A., Kinnunen P. K. Triggering of the activity of phospholipase A2 by an electric field. Biochemistry. 1987 Aug 11;26(16):4907–4910. doi: 10.1021/bi00390a002. [DOI] [PubMed] [Google Scholar]
- White S. H., Jacobs R. E., King G. I. Partial specific volumes of lipid and water in mixtures of egg lecithin and water. Biophys J. 1987 Oct;52(4):663–665. doi: 10.1016/S0006-3495(87)83259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D. H., Brockman H. L. Regulation of the surface pressure of lipid monolayers and bilayers by the activity of water: derivation and application of an equation of state. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4285–4289. doi: 10.1073/pnas.85.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- de Kroon A. I., de Gier J., de Kruijff B. Association of synthetic model peptides with phospholipid vesicles induced by a membrane potential. Biochim Biophys Acta. 1989 Jun 6;981(2):371–373. doi: 10.1016/0005-2736(89)90051-5. [DOI] [PubMed] [Google Scholar]


