Abstract
To further explore the nature of the mitochondrial dysfunction and insulin resistance that occur in the muscle of young, lean, normoglycemic, insulin-resistant offspring of parents with type 2 diabetes (IR offspring), we measured mitochondrial content by electron microscopy and insulin signaling in muscle biopsy samples obtained from these individuals before and during a hyperinsulinemic-euglycemic clamp. The rate of insulin-stimulated muscle glucose uptake was approximately 60% lower in the IR offspring than the control subjects and was associated with an approximately 60% increase in the intramyocellular lipid content as assessed by 1H magnetic resonance spectroscopy. Muscle mitochondrial density was 38% lower in the IR offspring. These changes were associated with a 50% increase in IRS-1 Ser312 and IRS-1 Ser636 phosphorylation and an approximately 60% reduction in insulin-stimulated Akt activation in the IR offspring. These data provide new insights into the earliest defects that may be responsible for the development of type 2 diabetes and support the hypothesis that reductions in mitochondrial content result in decreased mitochondrial function, which predisposes IR offspring to intramyocellular lipid accumulation, which in turn activates a serine kinase cascade that leads to defects in insulin signaling and action in muscle.
Introduction
Recent magnetic resonance spectroscopy (MRS) studies have revealed increased intramyocellular lipid content associated with reduced mitochondrial phosphorylation activity in the muscle of young, lean, normoglycemic, insulin-resistant offspring of parents with type 2 diabetes (IR offspring) (1). These data suggest a potential role of mitochondrial dysfunction in the pathogenesis of insulin resistance and type 2 diabetes; however, the underlying mechanism responsible for this reduced mitochondrial activity remains unknown.
Increases in the intramyocellular concentration of fatty acid metabolites have been postulated to activate a serine kinase cascade, causing increased phosphorylation of IRS-1 on critical serine sites, which blocks insulin receptor phosphorylation of IRS-1 on tyrosine sites. This results in reduced insulin-stimulated IRS-1–associated PI3K activity (2–5), decreased insulin-stimulated glucose transport activity (3), and reduced muscle glycogen synthesis (6, 7). However, there is currently little evidence that serine phosphorylation of IRS-1 is a key molecular event for this process in humans or whether or not there are associated alterations in insulin-stimulated Akt activity, an essential step for insulin stimulation of glucose transport in skeletal muscle.
The purpose of this study was 2-fold. First, in order to examine the mechanism responsible for the reduction of mitochondrial activity in young, lean, insulin-resistant offspring of parents with type 2 diabetes, we analyzed mitochondrial density in muscle biopsy samples by electron microscopy and analyzed the expression of several key transcriptional factors and coregulators that are known to regulate mitochondrial biogenesis, including PPARγ coactivator 1α (PGC-1α), PGC-1β, nuclear respiratory factor–1 (NRF-1), NRF-2, and mitochondrial transcription factor A (mtTFA). Second, in order to assess the potential role of IRS-1 serine phosphorylation in the pathogenesis of insulin resistance, we also examined IRS-1 serine phosphorylation on several serine residues (Ser307, Ser312, Ser616, Ser636) that have previously been implicated to interfere with insulin signaling in vitro. The subjects in this cohort were selected to examine these questions since, in contrast to patients with diabetes, they are young, lean, healthy, and do not have other confounding factors that are typically associated with obesity and diabetes.
Results
Subject characteristics.
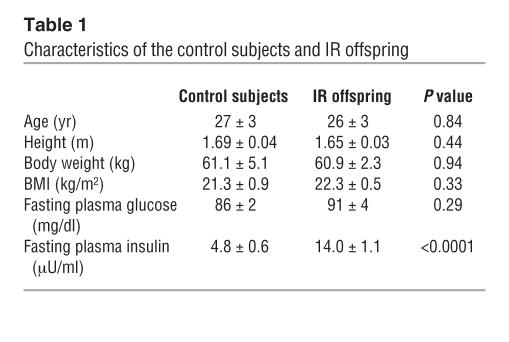
Insulin-sensitive control subjects and IR offspring were group matched for age, weight, height, BMI, and activity index (Table 1). The insulin sensitivity index was markedly lower in the IR offspring than in the control subjects (2.8 ± 0.2 vs. 10.2 ± 0.7; P < 0.00001).
Table 1.
Characteristics of the control subjects and IR offspring
Oral glucose tolerance test.
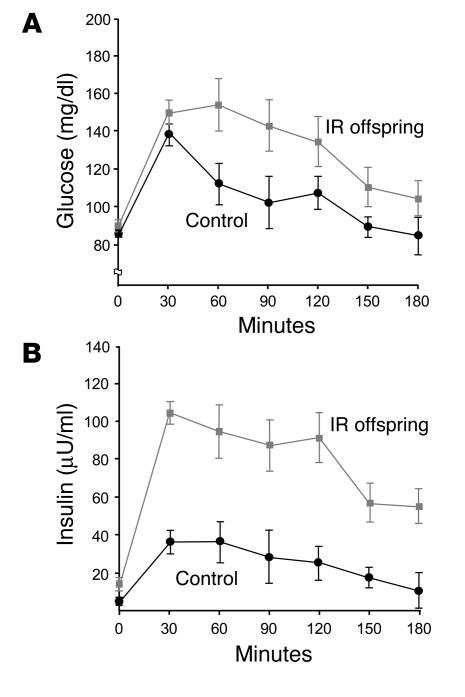
All subjects had normal glucose tolerance test results, but plasma concentrations of glucose (Figure 1A) and insulin (Figure 1B) during the test were significantly higher in IR offspring than control subjects (P < 0.05 and P < 0.001, respectively). Fasting plasma fatty acid concentrations were similar in control subjects (0.53 ± 0.10) and IR offspring (0.50 ± 0.05; P = 0.76) and decreased by approximately 90% in both groups during the glucose tolerance test.
Figure 1.
Results of oral glucose tolerance test. Mean plasma concentrations of glucose (A) and insulin (B) before and during a 75-g oral glucose tolerance test in control subjects (n = 6) and IR offspring (n = 8). P = 0.0009 for the comparison of the areas under the curve for insulin concentration of control subjects and IR offspring.
Hyperinsulinemic-euglycemic clamp studies.
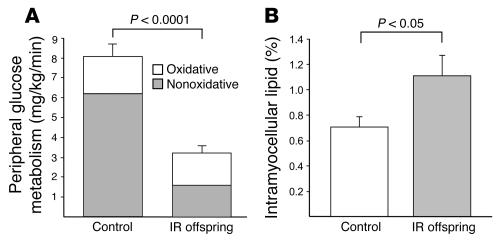
Fasting rates of glucose production were similar in control subjects (2.3 ± 0.2 mg/kg/min) and IR offspring (2.0 ± 0.1 mg/kg/min; P = 0.10) and were completely suppressed in both groups during the hyperinsulinemic-euglycemic clamp. In contrast, rates of glucose infusion required to maintain euglycemia were 58% lower in IR offspring than in control subjects during the clamp (7.9 ± 0.6 mg/kg/min vs. 3.3 ± 0.4 mg/kg/min; P < 0.0001), and insulin-stimulated rates of peripheral glucose uptake were also approximately 60% lower in IR offspring (P < 0.0001; Figure 2A). This reduction in peripheral glucose metabolism could be attributed almost entirely to an approximately 70% reduction in non-oxidative glucose disposal in the IR offspring.
Figure 2.
Hyperinsulinemic-euglycemic clamp and intramyocellular lipid content. (A) Insulin-stimulated rates of muscle glucose metabolism in control subjects (n = 6) and IR offspring (n = 8). (B) Intramyocellular lipid content in soleus muscle of control subjects (n = 6) and IR offspring (n = 8), measured by localized 1H MRS.
Intramyocellular triglyceride content.
Intramyocellular lipid content, measured by localized 1H MRS of the soleus muscle, was approximately 60% higher in IR offspring than in control subjects (Figure 2B).
Mitochondrial density.
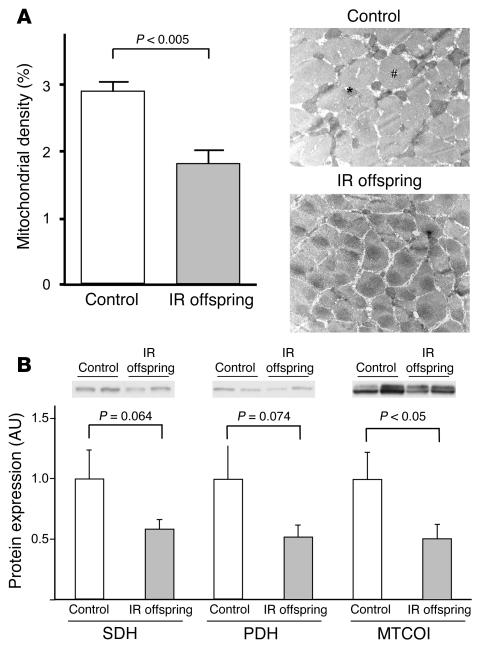
Mitochondrial density, assessed by electron microscopy, was found to be 38% (P < 0.005) lower in IR offspring than control subjects (Figure 3A). We also analyzed the expression of several key mitochondrial- and nuclear-encoded proteins that are involved with energy metabolism in the mitochondria. Expression of cytochrome c oxidase I (MTCOI), which is coded by the mitochondrial genome, was decreased by 50% in IR offspring (P < 0.05). Pyruvate dehydrogenase (PDH) and succinate dehydrogenase (SDH), which are both encoded by the nuclear genome, tended to be decreased in IR offspring (Figure 3B).
Figure 3.
Mitochondrial density and gene expression data. (A) Mitochondrial density in control subjects (n = 6) and IR offspring (n = 8), assessed by electron microscopy. The pound symbol (#) indicates muscle fiber; the asterisk indicates mitochondrion. (B) Mitochondrial protein expressions assessed by Western blotting in control subjects (n = 6) and IR offspring (n = 9). Results were normalized to β-actin protein expression. SDH, succinate dehydrogenase; PDH, pyruvate dehydrogenase.
Expression of key transcriptional regulatory factors and cofactors that regulate mitochondrial biogenesis.
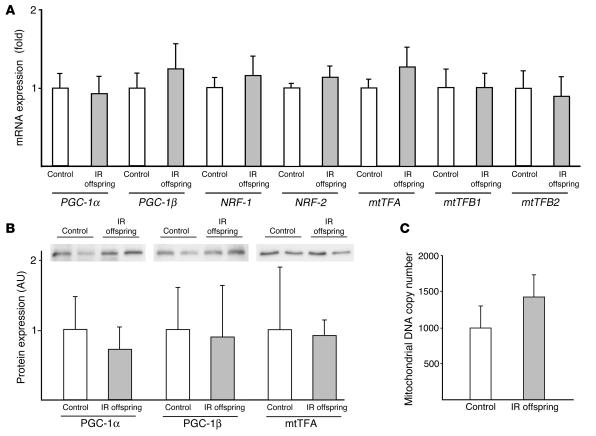
To examine potential factors that may be responsible for the reduced mitochondrial content observed in the IR offspring, we analyzed mRNA of PGC-1α and PGC-1β by real-time quantitative PCR. We found no differences between groups in mRNA expression of either of these factors. There were also no differences between the 2 groups in the expression level of mRNA for NRF-1, NRF-2, and mtTFA or protein expression of PGC-1α, PGC-1β, and mtTFA (Figure 4, A and B). We also measured mitochondrial DNA (mtDNA) copy number in skeletal muscle using quantitative PCR and did not observe any differences in this parameter between the 2 groups (Figure 4C).
Figure 4.
mRNA and protein expression of mitochondrial biogenesis genes. (A) mRNA expression of PGC-1 and downstream genes determined by real-time quantitative PCR using a TaqMan probe in control subjects (n = 7) and IR offspring (n = 13). (B) Protein expression of PGC-1α, PGC-1β, and mtTFA measured by Western blotting in control subjects (n = 6) and IR offspring (n = 9). (C) mtDNA copy number was determined by real-time quantitative PCR using a TaqMan probe against NADH dehydrogenase 2 (ND2) and β-actin. mtDNA copy number was calculated as the ratio of ND2 to β-actin in control subjects (n = 7) and IR offspring (n = 9).
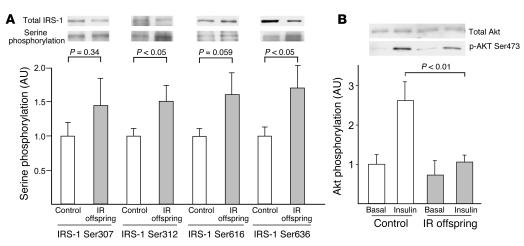
IRS-1 serine phosphorylation and Akt activation.
Basal IRS-1 serine phosphorylation at Ser312 and Ser636 residues was increased by approximately 50% in IR offspring (Figure 5A). There was also a strong tendency toward increased phosphorylation at the Ser616 residue of IRS-1 in the IR offspring. There was no difference in IRS-1 expression between the 2 groups. We also examined insulin activation of Akt, a key step in insulin-stimulated glucose transport activity, by measuring Akt phosphorylation with Western blotting and found that it was reduced by approximately 60% in the IR offspring (Figure 5B).
Figure 5.
Insulin signaling data. (A) IRS-1 serine phosphorylation at Ser307, Ser312, Ser616, and Ser636 in the basal state in control subjects (n = 10) and IR offspring (n = 7). (B) Insulin-stimulated Akt phosphorylation at 20 minutes on Ser473 in control subjects (n = 5) and IR offspring (n = 7). Akt phosphorylation was normalized to total Akt protein expression.
Discussion
Previously, we found that mitochondrial ATP production in skeletal muscle was decreased by approximately 30% in a similar cohort of young, lean IR offspring (1). We hypothesized that this reduction in mitochondrial oxidative phosphorylation might be responsible for the increased intramyocellular lipid content and associated muscle insulin resistance in these individuals (1, 7). To further examine the mechanism responsible for reduced mitochondrial activity in these subjects, we assessed mitochondrial density by electron microscopy in the present study and found that mitochondrial density was reduced by 38% in the IR offspring. In agreement with this finding, we also measured the expression of several mitochondrial proteins and found MTCOI to be reduced by approximately 50% in IR offspring, with a tendency for succinate dehydrogenase and pyruvate dehydrogenase to be reduced by a similar amount. These data suggest that IR offspring may have an inherited condition that causes a reduction in mitochondrial content in skeletal muscle, which in turn may be responsible for the reduced rates of mitochondrial oxidative phosphorylation.
To examine factors that might be responsible for the reduced mitochondrial content in IR offspring, we also assessed expression of several key regulators of mitochondrial biogenesis in their skeletal muscle. PGC-1α and PGC-1β are transcriptional cofactors that regulate mitochondrial biogenesis. Two recent DNA microarray studies found a coordinated reduction in the expression of genes encoded by PGC-1α in the skeletal muscle of type 2 diabetic patients (8, 9) and overweight nondiabetic subjects with a family history of diabetes (9). However, in contrast to these studies, we did not observe any difference in the expression level of mRNA or protein content of either PGC-1α or PGC-1β. Furthermore, we also examined the mRNA expression of NRF-1, NRF-2, and mtTFA, which have been shown to be involved in mitochondrial biogenesis and are regulated by PGC-1, and found no difference between the groups in the level of mRNA expression of these factors. The reason for the disparity between our findings and those of previous studies are not clear. While it is possible that it may be related to the smaller sample size of the present study, it may also be related to the fact that our IR offspring were young, lean, and healthy, in contrast to the subjects in the other studies, who were older, obese, and diabetic (8, 9) or, in the case of the first-degree nondiabetic relatives, overweight (9). Indeed, a recent study by Ling et al. has demonstrated an age-dependent decrease in muscle gene expression of PGC-1α and PGC-1β in young and elderly dizygotic and monozygotic twins without known diabetes (10). Interestingly, despite the observed reductions in mitochondrial density in the IR offspring, we did not observe any differences in the mtDNA content between the 2 groups. These data are consistent with the lack of differences in the expression levels of PGC-1α, PGC-1β, NRF-1, and mtTFA and taken together suggest that there are other unknown factors involved in the regulation of mitochondrial biogenesis that are responsible for the reduced skeletal muscle mitochondrial content in the IR offspring.
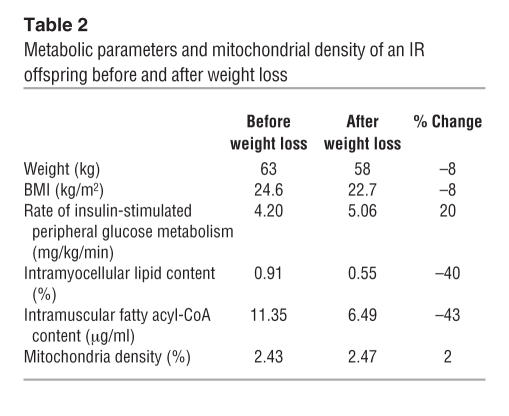
Previous studies have shown that insulin per se stimulates mitochondrial biogenesis (11, 12) and that mitochondrial morphology is altered in obese insulin-resistant and type 2 diabetic individuals (13). It is therefore possible that insulin resistance is the cause of the reduced mitochondrial content that we observed in the IR offspring, as opposed to being the result. To investigate this possibility, we examined mitochondrial content, intramyocellular lipid content, and insulin action in an insulin-resistant individual before and after a 5-kg weight loss. Following this modest weight loss, insulin-stimulated muscle glucose disposal increased by approximately 20% and was associated with a 40% decrease in intramyocellular lipid content and a 43% reduction in intramuscular long-chain fatty acyl-CoA content. However, these changes in insulin resistance and intramyocellular lipid content were unassociated with any alterations in the mitochondrial density, suggesting that reductions in mitochondrial content predispose individuals to dysregulated intramyocellular lipid metabolism and insulin resistance. In addition, we did not observe any differences in expression of PGC-1α or PGC-1β (or downstream PGC-1–regulated genes) between the control and insulin-resistance subjects, suggesting that the observed reductions in mitochondrial density are unlikely due to insulin- or fatty acid–induced alterations in PGC-1α or PGC-1β expression.
Insulin resistance in the IR offspring could mostly be attributed to a 70% reduction in insulin-stimulated non-oxidative muscle glucose metabolism, associated with a reduction in insulin-stimulated muscle glucose transport activation (14, 15). Insulin-stimulated Akt activation has been shown to be a key step for the activation of glucose transport in skeletal muscle. In this study we found that insulin activation of Akt phosphorylation was reduced by approximately 60% in the IR offspring. Using 1H MRS to measure intramyocellular lipid content, we found that insulin resistance in muscle of IR offspring was accompanied by an approximately 60% increase in intramyocellular lipid content, as compared with insulin-sensitive control subjects. These data are consistent with those of previous studies in humans (7, 16, 17) and rodents (18, 19), which have suggested that dysregulated intramuscular fatty acid metabolism has an important causative role in insulin resistance and may have a similar role in fat-induced insulin resistance in the skeletal muscle of insulin-resistant offspring. It has previously been hypothesized that intracellular fatty acid metabolites activate a serine kinase cascade that leads to increased phosphorylation of IRS-1 on critical serine residues, which, in turn, blocks insulin receptor kinase phosphorylation of IRS-1 on critical tyrosine sites that are required for PI3K association and activation (20). Evidence in support of this hypothesis stems from in vitro studies demonstrating that mutating IRS-1 Ser307 to Ala307 abrogates TNF-1α–induced insulin resistance in 32DIR cells (21). To examine this hypothesis in human skeletal muscle, we assessed phosphorylation of several IRS-1 serine sites that have been suggested to cause insulin resistance in vitro and in animal models (22–27). Consistent with this hypothesis, we found an approximately 50% increase in the amount of IRS-1 Ser312 and IRS-1 Ser636 phosphorylation in muscle biopsy samples obtained from IR offspring following an overnight fast. In addition, there was a strong tendency toward increased phosphorylation of Ser616 in the IR offspring compared with control subjects.
In summary, these results provide new insights into the earliest defects that may be responsible for the pathogenesis of type 2 diabetes. These data support the hypothesis that reductions in mitochondrial content are at least in part responsible for the reduced mitochondrial activity that has previously been described in IR offspring. Furthermore, these changes are independent of alterations in the expression levels of PGC-1α, PGC-1β, NRF-1, and mtTFA, suggesting that there are other unknown factors responsible for the reduced mitochondrial content in the IR offspring. Finally, we demonstrate that the IR offspring have reduced insulin-stimulated Akt activation associated with increased phosphorylation of IRS-1 Ser312 and Ser636, which may explain their profound defect in insulin-stimulated muscle glucose metabolism.
Methods
Subjects.
All subjects were recruited by means of local advertising and were prescreened to confirm that they were in excellent health, lean, nonsmoking, and taking no medications. A birth weight higher than 2.3 kg and a sedentary lifestyle, as defined by an activity index questionnaire (28), were also required. Qualifying subjects underwent a 3-hour oral glucose tolerance test (with a 75-g oral glucose load), after which 2 subgroups of subjects were selected based on extreme phenotypes for insulin resistance and increased insulin sensitivity.
Insulin-resistant subjects (1 male and 7 female) were defined as having an insulin sensitivity index of less than 4.0, at least 1 parent or grandparent with type 2 diabetes, and at least 1 other family member with type 2 diabetes (29). Insulin-sensitive control subjects (3 male and 3 female) were defined as having an insulin sensitivity index greater than 7.0 and no family history of type 2 diabetes.
All qualifying subjects underwent a complete medical history evaluation and a physical examination along with blood tests to verify that the following were normal: blood and platelet counts; concentrations of electrolytes, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, cholesterol, and triglycerides; prothrombin time; and partial thromboplastin time. In addition, 1H MRS was used to measure the triglyceride content of the gastrocnemius/soleus muscle. The subjects then underwent a hyperinsulinemic-euglycemic clamp study to assess the responsiveness of liver and muscle.
Written consent was obtained from each subject after the purpose, nature, and potential complications of the studies had been explained. The protocol was approved by the Yale University School of Medicine Human Investigation Committee.
Diet and study preparation.
For 3 days before the studies (oral glucose tolerance test, clamp, and MRS), the subjects were instructed to eat a regular, weight-maintenance diet containing at least 150 g of carbohydrate and not to perform any exercise other than normal walking for the 3 days before the study. To minimize changes in insulin sensitivity resulting from ovarian hormonal effects, female subjects were studied during the follicular phase (days 0 through 12) of the menstrual cycle (30). Subjects were admitted to the Yale–New Haven Hospital General Clinical Research Center the evening before the clamp study, and the subjects continued to fast while having free access to regular drinking water until the completion of the study the following day.
Measurement of metabolites and hormones.
Plasma glucose concentrations were measured with a YSI 2700 STAT Analyzer (YSI Inc.). Plasma concentrations of insulin were measured using double-antibody radioimmunoassay kits (Linco Research Inc.). Plasma fatty acid concentrations were determined by a microfluorometric method (31). Gas chromatography/mass spectrometry analyses of the enrichment of [6,6-2H2]glucose in plasma were measured with a Hewlett-Packard Mass Selective Detector (model 5971A) as previously described (32). Intramuscular fatty acyl-coA content was measured by tandem mass spectrometry as previously described (4).
MRS of intramyocellular triglyceride content.
On a separate day, all subjects were transported by wheelchair to the Yale Magnetic Resonance Center, and localized 1H MRS spectra of the soleus muscle was acquired on a 2.1-T BioSpec Spectrometer (Bruker BioSpin Corp.) as previously described (32).
Hyperinsulinemic-euglycemic clamp studies.
Basal rates of glucose turnover were assessed during a 3-hour basal period, and insulin-stimulated rates were assessed with a 3-hour hyperinsulinemic-euglycemic clamp with the infusion of 20 mU of insulin (U-100 regular insulin; Novo Nordisk) per square meter of body surface area per minute and using [6,6-2H2]glucose as previously described (33).
Muscle biopsy.
During the basal period of glucose tracer infusion during the clamp, the skin over the vastus lateralis muscle was sterilely prepared with Betadine, and 1% lidocaine was injected subcutaneously. A 2-cm incision was made using a scalpel, and a baseline punch muscle biopsy was extracted using a 5-mm Bergstrom biopsy needle (Depuy Co.). A piece of muscle tissue was dissected with a scalpel and immediately fixed in glutaraldehyde buffer for electron microscopy studies as described below. The remainder of the muscle tissue was blotted, snap-frozen, and stored in liquid nitrogen until assay. The hyperinsulinemic-euglycemic clamp was begun after this baseline muscle biopsy, as described above. After 20 minutes of hyperinsulinemia, a repeat punch muscle biopsy was performed in the same site as the basal biopsy to assess insulin signaling. Given the small amount of tissue that we were able to obtain from some of the needle biopsy studies, we were occasionally limited in the amount of assays we could perform on any particular sample. Our first priority was to have enough tissue to assess mitochondrial content by electron microscopy, which was performed on all subjects represented in Tables 1 and 2 and Figures 1, 2, and 3A. In order to increase the power to detect differences in insulin signaling, protein and mRNA expression levels (Figures 3B, 4, and 5), we recruited additional IR offspring (n = 7) and control subjects (n = 5) who met the same inclusion and exclusion criteria as the other participants (insulin sensitivity index: IR offspring, 2.5 ± 0.3, control subjects, 10.4 ± 1.4; insulin-stimulated peripheral glucose metabolism: IR offspring, 2.8 ± 0.4 mg/kg/min, control subjects, 7.8 ± 0.3 mg/kg/min).
Table 2.
Metabolic parameters and mitochondrial density of an IR offspring before and after weight loss
Transmission electron microscopy.
For electron microscopic examination, individual muscle samples were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4°C overnight, postfixed in 1% osmium tetroxide in the same buffer for 1 hour at room temperature, stained in 2% uranyl acetate, dehydrated in a series of ethanol dilutions (50–100%), and embedded in epoxy resin (EMbed 812; Electron Microscopy Sciences). Ultrathin sections (60 nm) were stained with 2% uranyl acetate and lead citrate and examined in a Tecnai 12 BioTWIN electron microscope (FEI Co.). Three random sections were examined from each individual muscle, and 5 random pictures were taken from each section at a magnification of 6,800 and printed at a final magnification of 18,500. The volume density of mitochondria was estimated using the point-counting method in a blinded fashion. For each set of 5 pictures, average volume density was calculated and the mean of 3 values used to estimate the volume density for each individual muscle (34).
Real-time quantitative PCR analysis.
Total RNA was isolated from each basal muscle section using an RNeasy kit (QIAGEN). cDNA was prepared from 1 μg of RNA using the StrataScript RT-PCR kit (Strategene) with random hexamer primers, according to the manufacturer’s instructions. The resulting cDNA was diluted, and a 5-ng aliquot was used in 50 μl PCR reaction using a TaqMan gene expression assay (Applied Biosystems). PCR reactions were run in duplicate and quantitated with an ABI Prism 7700 Sequence Detection System (Applied Biosystems). Cycle threshold values were normalized to 18S ribosomal RNA expression, and results were expressed as a fold change in mRNA compared with insulin-sensitive control subjects.
mtDNA content.
Total DNA was isolated from 5 mg of each basal muscle section using a QIAamp DNA Micro Kit (QIAGEN). Five nanograms of total DNA was used as a template in 50 μl PCR reaction using a TaqMan gene expression assay against NADH dehydrogenase 2 (mitochondrial genome [ND2]) and β-actin (nuclear genome). mtDNA copy number was presented as a ratio of ND2 to β-actin as previously described (35).
Western blot and immunoprecipitation assay.
Western blot and immunoprecipitation assays were performed as described previously (36). Briefly, samples were homogenized in lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2 mM sodium vanadate, 100 mM NaF, 20 mM sodium pyrophosphate, 20 μg/ml aprotinin, and 1 mM PMSF. One milligram of lysate underwent immunoprecipitation by incubation with 4 μg of anti–IRS-1 antibody (Upstate) for 2 hours at 4°C, then with protein A/G agarose (Santa Cruz Biotechnology Inc.) overnight. The beads were washed 3 times before immunoblot analysis. Samples were denatured with Laemmli sample buffer for 5 minutes at 95°C, and the supernatant was separated using 10% SDS gel (Bio-Rad Laboratories Inc.) and electrotransferred onto PVDF membranes (Amersham Pharmacia Biotech Inc.). Samples were probed with anti-IRSp307, anti-IRSp312, anti-IRSp616, and anti-IRSp636 antibodies (Cell Signaling Technology). Images were analyzed and quantified with Quantity One (Bio-Rad Laboratories). To normalize data, the intensity of serine phosphorylation was divided by total IRS-1 protein expression, which was determined by Western blotting using anti–IRS-1 antibody.
Western blotting was performed as described previously (36). Forty micrograms of homogenized samples were blotted on a PVDF membrane. The membrane was probed with antibodies against phospho-Akt Ser473 (1:1,000; Cell Signaling Technology), total Akt (1:1,000; Cell Signaling Technology), IRS-1 (1:1,000; Upstate), PGC-1α and -1β (1:1,000; kindly provided by Bruce Spiegelman, Dana-Farber Cancer Institute and Department of Cell Biology, Harvard Medical School, Boston, Massachusetts, USA), mtTFA (1:500; Santa Cruz Biotechnology Inc.), PDH (1:3,000; Invitrogen Corp.), SDHA (SDH subunit A; 1:5,000; Abcam), and MTCOI (1:2,000; Abcam) overnight at 4°C. Equal protein loading was confirmed by reblotting of the membranes with a goat polyclonal antibody against β-actin (1:1,000; Santa Cruz Biotechnology Inc.).
Effect of weight loss.
In order to examine whether or not reductions in mitochondrial content might be secondary to increases in intramyocellular lipid content and/or insulin resistance, 1 insulin-resistant participant underwent a hypocaloric dietary regimen for approximately 7 weeks, without any change in physical activity, which resulted in an approximately 5-kg weight loss. Following weight stabilization, the subject underwent a repeat hyperinsulinemic-euglycemic clamp to assess muscle sensitivity, repeat 1H MRS to assess intramyocellular lipid content, and repeat muscle biopsy to assess mitochondrial content and intramuscular fatty acyl-CoA content (Table 2).
Statistics.
Statistical analyses were performed with JMPIN version 4.0.4 (SAS Institute). Statistically significant differences between control subjects and insulin-resistant subjects were detected using unpaired Student’s t tests (2-tailed) for independent samples. P < 0.05 was considered statistically significant. All data are expressed as mean ± SEM.
Acknowledgments
We are indebted to James Dziura for statistical assistance; to Yanna Kosover, Mikhail Smolgovsky, Anthony Romanelli, Irene Moore, Katherine Goheen, Christina Horensavitz, Alessandro De Camilli, Aida Groszmann, Andrea Belous, Jonas Lai, Sandra Alfano, and the staff of the Yale–New Haven Hospital General Clinical Research Center for expert technical assistance with the studies; and to the volunteers for participating in this study. This work was supported by NIH grants P01 DK-68229, R01 AG-23686, R01 DK-063192, R01 DK-49230, P30 DK-45735, and M01 RR-00125 and a Distinguished Clinical Scientist Award from the American Diabetes Association (to G.I. Shulman).
Footnotes
Katsutaro Morino and Kitt Falk Petersen contributed equally to this work.
Nonstandard abbreviations used: IR offspring, insulin-resistant offspring of parents with type 2 diabetes; MRS, magnetic resonance spectroscopy; MTCOI, mitochondria-encoded cytochrome c oxidase I; mtDNA, mitochondrial DNA; mtTFA, mitochondrial transcription factor A; NRF-1, nuclear respiratory factor–1; PGC-1α, PPARγ coactivator 1α.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin ME, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 3.Dresner A, et al. Effect of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 5.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 6.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J. Clin. Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perseghin G, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 8.Mootha VK, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 9.Patti ME, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling C, et al. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J. Clin. Invest. 2004;114:1518–1526. doi:10.1172/JCI200421889. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes. 2002;51:1913–1920. doi: 10.2337/diabetes.51.6.1913. [DOI] [PubMed] [Google Scholar]
- 13.Ritov VB, et al. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 14.Cline GW, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, et al. The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J. Clin. Invest. 1996;97:2705–2713. doi: 10.1172/JCI118724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krssak M, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am. J. Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 18.Kraegen EW, et al. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman GI. Cellular mechanisms of insulin resistance. J. Clin. Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguirre V, et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 22.De Fea K, Roth RA. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry. 1997;36:12939–12947. doi: 10.1021/bi971157f. [DOI] [PubMed] [Google Scholar]
- 23.Paz K, et al. Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J. Biol. Chem. 1999;274:28816–28822. doi: 10.1074/jbc.274.40.28816. [DOI] [PubMed] [Google Scholar]
- 24.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 25.Bouzakri K, et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- 26.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, et al. Prevention of fat-induced insulin resistance by salicylate. J. Clin. Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 30.Diamond MP, Jacob R, Connolly-Diamond M, DeFronzo RA. Glucose metabolism during the menstrual cycle: assessment with the euglycemic, hyperinsulinemic clamp. J. Reprod. Med. 1993;38:417–421. [PubMed] [Google Scholar]
- 31.Miles J, Glasscock R, Aikens J, Gerich J, Haymond MA. Microfluorometric method for the determination of free fatty acids in plasma. J. Lipid Res. 1983;24:96–99. [PubMed] [Google Scholar]
- 32.Mayerson AB, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggs DG, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1998;128:176–185. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Zong H, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhat HK, Epelboym I. Quantitative analysis of total mitochondrial DNA: competitive polymerase chain reaction versus real-time polymerase chain reaction. J. Biochem. Mol. Toxicol. 2004;18:180–186. doi: 10.1002/jbt.20024. [DOI] [PubMed] [Google Scholar]
- 36.Samuel VT, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]