Abstract
Encapsulated meningococci are complement sensitive only in the presence of bactericidal antibodies by yet-unexplored mechanisms. The objective of this study was to investigate the involvement of major bacterial surface constituents on complement activation and membrane attack complex (MAC) formation on serogroup B meningococci in the presence or absence of antibody-dependent serum bactericidal activity (SBA). The strains used were the encapsulated H44/76, five of its variants differing in capsulation and expression of the class 1 porin (PorA), and its lipopolysaccharide (LPS)-deficient isogenic mutant (LPS−) pLAK33. Two normal sera, one with high SBA (SBA+) and one with no bactericidal activity (SBA−) against H44/76 as well as an a-γ-globulinemic serum were used for sensibilization of the bacteria. C3b and iC3b deposition on H44/76, its unencapsulated variant v24, and pLAK33 was similar in SBA+ and SBA− serum, and no difference was present between the strains. MAC deposition on H44/76 was higher in SBA+ serum than in SBA− serum and the a-γ-globulinemic serum. The amounts of C3b on H44/76, v24, and pLAK33 in the a-γ-globulinemic serum were also not different, indicating immunoglobulin G (IgG)- and LPS-independent complement activation. H44/76 PorA(+) and its PorA(−) variant and the v24 PorA(+) and its PorA(−) variant incubated in SBA− serum induced comparable amounts of MAC, despite their different serum sensitivities. Complement formation on the surface of the bacteria occurred almost exclusively via the classical pathway, but the considerable amounts of Bb measured in the serum indicated alternative pathway activation in the fluid phase. We conclude that complement deposition on meningococci is, for the most part, independent of classical pathway IgG and is not influenced by the presence of PorA or LPS on the meningococcal surface. Addition of an anti-PorA chimeric antibody to the nonbactericidal normal serum, while promoting a dose-related bacterial lysis, did not influence the amounts of C3b, iC3b, and MAC formed on the bacterial surface. These findings support the hypothesis that proper MAC insertion rather than the quantity of MAC formed on the bacterial surface is of importance for efficient lysis of meningococci.
Neisseria meningitidis or meningococcus is a gram-negative bacterium that colonizes only the nasopharynx of humans. Approximately 5 to 10% of healthy individuals are carriers, mostly for 6 to 9 months, the highest carrier frequency being among teenagers and young adults (2, 6, 7). N. meningitidis may cause bacteremia, meningitis, or fulminant septicemia in a relative small proportion of carriers, with high rates of mortality and morbidity (25).
The pathogenesis of meningococcal infection is not clear, but there is evidence that N. meningitidis has evolved mechanisms evading recognition by the immune system (reviewed in reference 49). Structural components, such as the polysaccharide capsule and mechanisms like sialylation of lipopolysaccharides (LPS) or lipooligosaccharides, render meningococci inaccessible to complement. However, the presence of various specific antibodies against N. meningitidis or antibodies cross-reacting with bacterial surface components can mediate complement activation leading to phagocytosis or direct bacterial lysis (15, 16).
Isolates from cases are nearly always encapsulated, and meningococcal capsular polysaccharides (CPS) are used to serogroup the bacteria. CPS of most meningococcal serogroups are very immunogenic, and CPS-containing meningococcal vaccines are available. For serogroup B, which account for 50% or more of meningococcal infections in Europe and North America (25), no CPS-based vaccine could be developed because of the poor immunogenicity of its capsule (13, 50). Alternatively, vaccine development against this serogroup has focused on subcapsular structures able to elicit antibodies, such as outer membrane proteins (OMPs). Class 1 porin (PorA) is a major OMP with high antigenic variability used to serosubtype meningococci (14). Antibodies against OMPs, especially against PorA, are bactericidal, and OMP-based vaccines have already given encouraging results in clinical trials (8, 9, 32, 35, 36, 39, 42). However, given the high antigenic variability of PorA, the nature and specificity of the most effective bactericidal antibodies has still to be defined.
The terminal pathway of complement plays a central role in the lysis of meningococci, given that individuals deficient in one of the late complement components have an almost 600-fold-higher risk than healthy individuals to develop meningococcal disease (10, 12, 40). Data from research with Neisseria gonorrhoeae and Salmonella enterica serovar Typhimurium suggest that proper MAC insertion is important for efficient killing (23, 24). However, the exact mechanism by which the MAC is inserted into the neisserial cell wall is unknown.
In this study, we investigated the influence of cell wall constituents of serogroup B N. meningitidis on complement activation in terms of deposition of C3b, iC3b, and terminal MAC formation on the meningococcal surface in the presence or absence of serum bactericidal activity. We used serogroup B meningococcal strains different in capsulation, in expression of the class 1 porin (PorA), and in expression of LPS. The pathway of complement activation was assessed by measuring in sera with or without bactericidal meningococcal antibodies the final product C4d (for the classical pathway) and the final product Bb (for the alternative pathway).
MATERIALS AND METHODS
N. meningitidis strains.
The encapsulated serogroup B reference strain H44/76 (B:15 P:1.7,16) and its unencapsulated variant V24 were used. Variants of these strains with a high level of expression or deletion of the PorA OM were also used and designated H44/76 PorA(+), v24 PorA(+) and H44/76 PorA(−), v24 PorA(−), respectively. These strains were obtained from the collection of the Reference Laboratory for Bacterial Meningitis (RLBM), University of Amsterdam (kindly offered by C. Hopman) and have been described previously (44, 45). The engineered LPS-free H44/76-mutant pLAK33 (offered by P. van der Ley, RIVM, Bildhoven, The Netherlands) was also used. This mutant lacks the LpxA gene encoding the LpxA protein, which catalyzes the addition of 3-OH fatty acid to uridine-diphospho (UPD)-N-acetylglucosamine, the first step in lipid A biosynthesis (41). This mutant does not contain LPS, since LPS is anchored to the OM by lipid A.
Human sera.
Sera from two healthy donors (designated A and B) and one a-γ-globulinemic patient (kindly provided by T. Out, Department of Immunology, Academic Medical Centre, Amsterdam, The Netherlands) were used. Sera A and B were selected on the basis of their antibody-dependent serum bactericidal activity (SBA) against the encapsulated strain H44/76 as tested in a bactericidal test (see below). Serum A was highly bactericidal against strain H44/76 and its PorA(+) and PorA(−) variants at a low concentration in serum (2%) while serum B and the a-γ-globulinemic serum showed no SBA, even at a concentration of 50%. The three sera had a normal complement hemolytic activity for all complement pathways. Heat-inactivated serum A added to serum B induced SBA of serum B, indicating that SBA was strictly antibody dependent.
All sera were bactericidal (serum concentration of <2%) against the unencapsulated v24 and its PorA(+) and PorA(−) variants, as well as the pLAK33 mutant strain.
Anti-complement antibodies.
Bacteria-bound total C3b was detected with the polyclonal mouse anti-human C3c HRP-conjugated antibody (Dako, Glostrup, Denmark), which also reacts with C3b. For the detection of iC3b, a rat anti-human iC3b-neoantigen monoclonal antibody was used. This antibody (C3g-clone 9) binds to iC3b and C3dg and was kindly provided P. Lachmann, Cambridge, United Kingdom.
The presence of terminal complement complex was demonstrated using the mouse anti-human SC5-9 neoepitope-specific monoclonal antibody aE11 (33) offered by T. E. Mollnes, Bodø, Norway.
N. meningitidis anti-PorA antibody.
In order to study the effect of specific anti-PorA bacterial antibodies on complement deposition, we used an anti-meningococcal PorA chimeric (humanized) antibody, kindly offered by J. van de Winkel, Academic Hospital, Utrecht, Netherlands. This antibody consists of the mouse Fab MN12H2, which is highly complementary to the P1.16 PorA epitope contained in our reference strain, H44/76 (43), and a portion of human immunoglobulin G1 (IgG1) Fc.
SBA assay.
This test was performed as described previously (11) with minor modifications. In brief, sera were heat inactivated and a series of twofold dilutions in Dulbecco's phosphate-buffered saline (PBS) buffer was made on microtiter plates. Serum from a donor with no intrinsic bactericidal activity against meningococci and with normal complement function was used as the complement source. This serum was added to every well at a final concentration of 17%, and after addition of the bacteria (3.3 × 103 CFU/ml [final concentration]), the plates were incubated at 37°C for 1 h. Volumes of 25 μl of each well were taken at 0 and 60 min of incubation and were plated on chocolate agar plates. After overnight incubation at 37°C, colonies were counted. SBA was defined as the concentration of serum able to kill at least 50% of the initial bacterial inoculum after 60 min of incubation.
A modification of this test was used for the assessment of SBA after addition of the anti-PorA chimeric antibody: the serum was not heat inactivated, and instead of serial dilutions, only one concentration (final concentration, 75%) was used throughout the test. The serum was or was not supplemented with increasing concentrations of the anti-PorA antibody (0.5 to 10 μg/ml).
Complement activation assay.
Bacteria grown overnight on chocolate agar were cultured to mid-log phase in tryptic soy broth (Difco, Detroit, Mich.). After being washed three times with PBS containing 0.05% Tween 20 (Sigma, St. Louis, Mo.), they were incubated in undiluted serum (109 CFU/ml) at 37°C for 5, 15, and 30 min. At each time point (0, 5, 15, and 30 min), samples of 200 μl taken from the suspensions were put in ice for 1 min to stop complement activation. After centrifugation at 13,000 × g at 4°C for 5 min, the supernatant was carefully separated from the pellet and stored in aliquots at −70°C for the detection of complement activation products in the fluid phase (C4d, Bb, and SC5b-9). The pellet was washed twice with buffer consisting of 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 1% (wt/vol) glucose, 1.0 mM MgCl2, and 0.6 mM CaCl2 (PiCM) and resuspended in 0.05 M NaHCO3, pH 9.6, yielding a final concentration 108 CFU/ml of suspension. Recovery of bacteria, tested on enzyme-linked immunosorbent assays (ELISAs) with an anti-meningococcal serogroup B polyclonal antibody, was reproducible.
ELISA to assess complement product deposition on surface of meningococci.
Surface-bound complement products were measured using an enzyme immunoassay based on the technique of Gordon et al. (17). Immulon II ELISA plates (Dynex Technologies, Chantilly, Va.) were coated with the bacterial suspension obtained from the complement activation assay (100 μl/well) overnight at 4°C. After five washes with PBS-Tween 20, blocking buffer (PiCM plus 2.5% [vol/vol] human serum albumin, 150 μl/well) at room temperature was added for 30 min to bind nonspecific sites. Subsequently, for the detection of C3b, mouse anti-human C3c antibody diluted 1:3,000 in PBS-Tween 20 supplemented with 0.1% (wt/vol) gelatin was added (100 μl/well) and incubated at 37°C for 1 h. For the detection of iC3b, rat anti-human C3b antibody (clone 9) was added (diluted 1:2,000) and incubated at 37°C for 2 h. The terminal complexes were detected with the aE11 monoclonal antibody (0.2 μg/ml) after incubation at 37°C for 1 h. After incubation with the substrate, the reactions were stopped and optical densities (ODs) were measured at 405 nm with an ELISA reader (Anthos Labtec Instruments, Salzburg, Austria).
For controls, we used unopsonized bacteria and bacteria opsonized in serum containing 10 mM EDTA or in heat-inactivated (at 56°C for 30 min) serum. In order to selectively block the classical pathway, 10 mM EGTA (Sigma) with 4 mM MgCl2 was added to the serum. In the controls with heat-inactivated or EDTA serum, the C3b, iC3b, and MAC values remained unchanged throughout the 30-min incubation and were equal to those obtained with the normal sera at time zero, considered to be background values. The controls with unopsonized bacteria had ODs lower than 0.1.
For the detection of C4d, Bb, and SC5b-9 in serum, commercially available ELISA kits were used (Quidel, San Diego, Calif.) and tests were performed according to the manufacturer's instructions (instruction leaflets for the C4d fragment enzyme immunoassay, the Bb fragment enzyme immunoassay, and SC5b-9 [TCC] enzyme immunoassay; Quidel).
Statistics.
Mean values and standard deviations (SD) of independent experiments were calculated. Statistical significance between experiments under different conditions was evaluated by the one-tailed paired Student's t test.
RESULTS
Complement activation conditions.
Our first aim was to develop a method for the measurement of complement products potentially produced by both classical and alternative pathways on the surface of meningococci. A whole-cell ELISA with coated bacteria and subsequent application of serum was not an appropriate method, since a serum concentration higher than 10% produced high background reactions (not shown). At serum concentrations lower than 10% though, only classical pathway activation can be seen (47). Our method of bacterial complement activation in fluid medium and subsequent coating onto ELISA plates resulted in reproducible results without the hindrance of a high background signal (Fig. 1, means ± SD of at least three experiments). For the detection of the MAC on the bacterial surface, various anti-terminal complex monoclonal antibodies were tested, but they reacted with the nonopsonized meningococcal strains (data not shown). The anti-C5b-9 monoclonal antibody aE11 yielded the least aspecific binding and was used in our experiments.
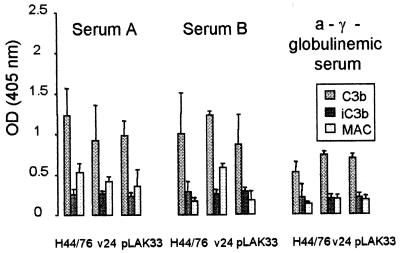
FIG. 1.
Deposition of C3b, iC3b, and MAC on cells of serogroup B meningococcal strain H44/76, its unencapsulated variant v24, and the LPS(−) mutant pLAK33 after 30 min of incubation with a serum with high bactericidal activity (serum A), a serum with no bactericidal activity (serum B), and one a-γ-globulinemic serum (means ± SD of at least three experiments). Values are ODs measured in whole-cell ELISAs with coated opsonized bacteria. ODs at time zero have been subtracted.
Total C3b, iC3b, and MAC deposition on serogroup B meningococcal strain H44/76 was quantified after opsonization with A, B, and a-γ-globulinemic sera at intervals of 0, 5, 15, and 30 min. In some initial experiments, bacteria were opsonized for up to 1 h. It was found that a gradual deposition of the complement components C3b, iC3b, and MAC on the bacteria occurred, reaching a peak at 30 min.
Complement deposition in presence or absence of natural bactericidal antibodies.
Incubation of H44/76 with the bactericidal serum A (Fig. 1) induced C3b deposition similar to that after incubation with the nonbactericidal serum B (Fig. 1, t test, P > 0.05). Incubation of H44/76 with the a-γ-globulinemic serum (Fig. 1) resulted in significantly less C3b (P < 0.01). When the unencapsulated variant v24 was used, C3b deposition was similar to that of H44/76 in all three sera (P > 0.1; Fig. 1). This indicated that the capsule was not a major factor in inhibiting C3b deposition on H44/76. C3b deposition on H44/76 and v24 in the a-γ-globulinemic serum indicated non-IgG-mediated complement activation. One possible factor that could have elicited non-IgG-mediated complement activation is LPS; therefore, we used the LPS-negative H44/76 mutant strain pLAK33. C3b deposited on cells of this strain in equal amounts as on cells of H44/76 and v24 in all three sera, indicating that bacterial constituents other than LPS can activate complement on meningococci in an immune serum as well as in a nonimmune serum.
Values of iC3b on the three strains were similar, irrespective of the serum used. iC3b was formed in small amounts, indicating that the major part of C3b deposited on meningococci was of the active form (Fig. 1).
MAC deposition on H44/76 was higher in the bactericidal serum A than in serum B or the a-γ-globulinemic serum (P < 0.01; Fig. 1). On strain v24 cells, MAC deposited in equal amounts after incubation in serum A and in serum B. When incubated in the a-γ-globulinemic serum, significantly less MAC was formed (P < 0.01), despite sensitivity of v24 in this serum. On pLAK33 MAC formation after incubation in serum A, serum B and the a-γ-globulinemic serum were not different.
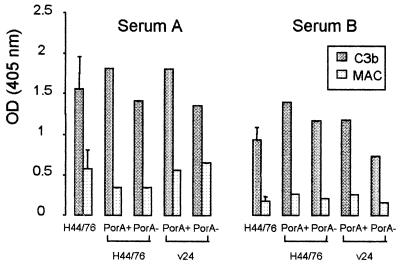
Since OMPs (porins) of gram-negative bacteria can activate complement in the absence of specific antibodies, we studied complement activation on H44/76 as well as on v24 isogenic PorA(+) and PorA(−) variants. C3b deposition on the PorA(+) or PorA(−) variants of either strain did not differ significantly in the two sera compared to deposition on cells of the H44/76 mother strain (Fig. 2). In the bactericidal serum A, MAC deposition was slightly higher (in two experiments) on the nonencapsulated v24 variants than on the encapsulated H44/76 variants. In the nonbactericidal serum B, MAC deposited similarly on the four variants although H44/76 PorA(+) and H44/76 PorA(−) were resistant to killing by this serum, in contrast to v24 PorA(+) and v24 PorA(−).
FIG. 2.
Deposition of C3b and MAC on cells of strain H44/76, its PorA(+) and PorA(−) variants, and the the PorA(+) and PorA(−) variants of the unencapsulated strain v24. Bacteria were incubated in a serum with high bactericidal activity (serum A) and a serum with no bactericidal activity (serum B).
Significant differences resulting from the above studies are summarized in Table 1.
TABLE 1.
Comparative results of SBA, C3b deposition, and MAC formation on various meningococcal strains exposed in the bactericidal serum A, the nonbactericidal serum B, and an a-γ-globulinemic serum
| N. meningitidis strains | Capsule presence | PorA expression | LPS presence | Findings in SBA− serum B compared to those in SBA+ serum Aa
|
Findings in a-γ-globulinemic serum compared to those in SBA+ serum A
|
||||
|---|---|---|---|---|---|---|---|---|---|
| SBA | C3b | MAC | SBA | C3b | MAC | ||||
| H44/76 | + | + | + | s↓ | n | s↓ | s↓ | s↓ | s↓ |
| V24 | − | + | + | n | n | n | n | n | s↓ |
| pLAK33 | + | + | − | n | n | n | n | n | s↓ |
| H44/76 PorA+ | + | ++ | + | s↓ | n | n | s↓ | ND | ND |
| H44/76 PorA− | + | − | + | s↓ | n | n | s↓ | ND | ND |
| V24 PorA+ | − | ++ | + | n | n | ↓b | n | ND | ND |
| V24 PorA− | − | − | + | n | n | ↓ | n | ND | ND |
s↓, significantly lower. n, no significant difference. ND, not done.
Results of two experiments.
Addition of chimeric anti-PorA antibody.
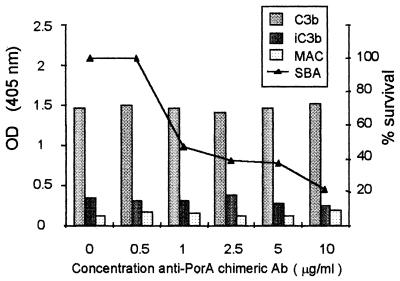
Increasing concentrations of the anti-PorA chimeric antibody ranging from 0.5 to 10 μg/ml were added to nonbactericidal serum B and, subsequently, the SBA against strain H44/76 PorA(+) was assessed in an SBA test. In parallel, the supplemented serum was used to opsonize H44/76 PorA(+) cells and to determine C3b, iC3b, and MAC deposition on their surfaces. The SBA increased in a dose-related manner (Fig. 3, means of two experiments). After addition of 1 μg of anti-PorA antibody/ml, less than 50% of CFU of the test strain survived, and after addition of 10 μg/ml, survival was less than 20%. The amounts of C3b, iC3b, and MAC on H44/76 PorA(+) cells incubated in the supplemented serum remained the same as in the nonsupplemented serum B. The amounts of C4d, Bb, and S5b-9 in the supplemented serum remained also unchanged (not shown).
FIG. 3.
Deposition of C3b, iC3b, and MAC on cells of strain H44/76 PorA(+) opsonized in nonbactericidal serum B supplemented with an anti-PorA chimeric antibody (means of two experiments). The concentrations of antibody used were 0.5 to 10 μg/ml. When supplemented with 1 μg of antibody/ml, the serum was able to kill more than 50% of the initial bacterial inoculum.
Pathway of complement activation on meningococcal surface.
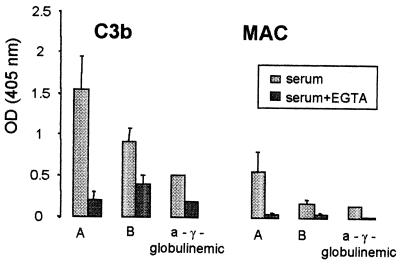
Incubation of H44/76 cells in sera supplemented with Mg2+ EGTA to block the classical pathway resulted in deposition of very small amounts of C3b and practically no formation of MAC (Fig. 4, mean values and SD of three experiments; values of the a-γ-globulinemic serum are of one experiment because of the scarcity of this serum). Similar results were obtained when the unecapsulated variant v24 or the four PorA(+) and PorA(−) variants were used (not shown).
FIG. 4.
Deposition of C3b and MAC on cells of strain H44/76 after incubation with a serum with high bactericidal activity (serum A), a serum with no bactericidal activity (serum B), and one a-γ-globulinemic serum, which was or was not supplemented with Mg2+ EGTA.
Pathway of complement activation in bactericidal and nonbactericidal serum.
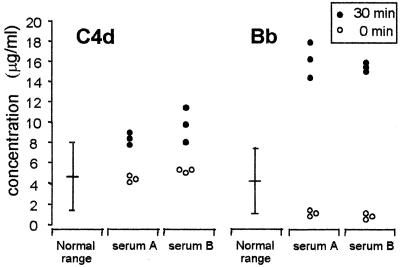
In serum A, concentrations of up to 48 μg/ml of nonlytic (vitronectin-bound) SC5b-9 could be measured after 30 min of incubation with the H44/76 strain while concentrations of up to 18.8 μg/ml were measured in the nonbactericidal serum B, indicating a higher overall complement activation in the bactericidal serum. The values of C4d and Bb in serum A and serum B at time zero and after incubation for 30 min with strain H44/76 are given in Fig. 5 and are the results of three independent experiments. C4d values in serum A and B increased just above the 2-SD range of the C4d values in normal nonactivated serum (instruction leaflet for C4d fragment enzyme immunoassay; Quidel), indicating activation of the classical pathway (34). Bb values in serum A as well as in serum B were clearly higher than the 2-SD range of the Bb values in normal nonactivated serum (instruction leaflet for Bb fragment enzyme immunoassay; Quidel), indicating activation of the alternative pathway (27).
FIG. 5.
Concentrations of complement products C4d and Bb in a bactericidal serum (serum A) and in a nonbactericidal serum (serum B) after 0 and 30 min of incubation with strain H44/76. Results are from three independent experiments. Normal ranges for data of C4d and Bb were previously determined.
DISCUSSION
Most studies involving the evasion of host defense mechanisms by N. meningitidis so far have been focused on the interactions between C3 and meningococcal surface components, such as CPS, LPS, and sialic acid (26, 30, 46-48). The capsule and, to a lesser degree LPS, sialylation appeared to be most important for complement resistance of meningococci (reviewed in references 38 and 49). However, encapsulated meningococci become serum sensitive if bactericidal antibodies against meningococcal CPS, LPS, or OMPs are present in the serum. We studied the complement activation by meningococcal capsule, LPS, and the PorA OMP by using appropriate mutants and human serum with or without bactericidal activity.
Incubation of H44/76 in a bactericidal or a nonbactericidal serum induced similar C3b deposition on the surface of this strain as well as of its unencapsulated (serum sensitive) variant. Although the degree of C3b deposition has been associated with bacterial lysis of various gram-negative bacteria (1, 31), we found no association between C3b deposition on meningococci and SBA, supporting the findings of others (20, 30, 47).
In order to investigate further the mediation of C3b formation on our meningococcal strains, we used an a-γ-globulinemic serum. In this serum, C3b deposition, even on the serum-resistant encapsulated strain, occurred, although to a lesser degree than in the bactericidal and the nonbactericidal serum, implying non-IgG-mediated complement activation. This of course cannot exclude IgM and IgA, which can still be present in an a-γ-globulinemic serum. Complement activation on H44/76 and its unencapsulated mutant occurred mainly via the classical pathway, since EGTA inhibited complement deposition. Jarvis and Vedros (19) found that addition of immune horse IgG antibodies to a nonbactericidal serum increased the alternative-pathway-mediated killing of N. meningitidis B16B6 in association with increased C3b deposition on the bacteria. They suggested that these IgG antibodies mediated the enhancement of C3 convertase formation. However, we found no greater alternative-pathway-mediated C3b deposition on our strains incubated in the bactericidal serum.
In order to analyze further the bacterial components that might have served as mediators for classical pathway complement activation, we used an LPS-negative strain. LPS has been referred to as a bacterial structure that can activate the classical pathway of complement without the mediation of IgG (28). The pLAK33 strain we used lacks the gene encoding lipid A, which anchors the LPS on the meningococcal surface, practically making this strain LPS negative. After incubation of PLAK33 in the a-γ-globulinemic serum, C3b was deposited on this strain in amounts similar to those on the LPS-intact strains. This indicates that LPS is not the major component for classical pathway complement activation on meningococci. However, it cannot be excluded that conformational changes on the membrane after removal of such a major component expose other epitopes able to activate complement or facilitate complement deposition. Since the LPS-negative strain—which retains its capsule—was serum sensitive in all three sera used, LPS appears to be indispensable for serum resistance of meningococci.
Another question we addressed was whether the difference in serum sensitivity between meningococci reflected inactivation of C3b (iC3b). Commercially available monoclonal antibodies to iC3b cannot exclusively distinguish for iC3b, since they also recognize the b-chain of active C3b. Therefore we used a monoclonal antibody that recognizes only iC3b and C3dg, thus excluding cross-reactions with the active C3b. We found that iC3b formation on H44/76 cells in the two sera with no SBA was not different than that in the bactericidal serum. Furthermore, there were no differences between serum-sensitive and serum-resistant strains. These findings indicate that lack of efficient lysis is not because of inactivation of C3b.
Ultimate lysis of meningococci occurs after insertion of the MAC into the bacterial surface. We found no association between C3b deposition on the meningococci and bacterial lysis, but this was not unexpected. C3b deposits randomly on its target sites at the cell wall, but in order to elicit the terminal complex formation, it has to bind to the C3b convertases (3, 5). Binding of C3b with C3 convertases transforms it to C5 convertase, cleaving C5 and initiating the assembly of MAC (37). Although C3b molecules bound to sites other than the convertases cannot be transformed to C5 convertases, they can still serve other processes, such as opsonization, leading to phagocytosis (5). Thus, only a minor proportion of C3b is involved in the formation of the terminal complement complex. It has been shown that C5b-7 dissociated from serum-resistant Salmonella minnesota after the addition of C8 and C9 while it remained bound on cells of the serum-sensitive strain (22). In contrast, serum-resistant and serum-sensitive gonococcal strains bound equal amounts of radiolabeled C5 and C9 at a ratio indicating that these molecules were incorporated into MAC (18). In our experiments, although it appeared that more MAC was formed on the encapsulated strain in the serum with high SBA, there were always measurable amounts of MAC formed on this strain when the nonbactericidal sera were also used. Furthermore, no large amounts of the nonlytic (vitronectin-bound) SC5b-9 (21, 37) were found in the nonbactericidal sera after activation. This indicates that no dissociation of MAC from the membrane occurred, although small amounts of nonlytic SC5b-9 could also bind on the bacterial cell wall (4). Since the lytic and nonlytic form of the terminal complex cannot be discriminated by the available monoclonal antibodies, it is unknown whether the lytic form of MAC was present on the bacterial surface. Measurements of vitronectin may possibly be worthwhile in order to assess the presence of the lytic form of MAC.
OMPs of gram-negative bacteria can also activate complement in the absence of specific antibodies (29). The class 1 porin (PorA) is a major meningococcal OMP that has gained much attention in vaccine design because of its effectiveness in inducing bactericidal antibodies (15, 16). However, PorA-deleted variants have been isolated from patients with meningococcal disease, and it has been suggested that the rise of such variants could be yet another mechanism for host immune evasion (44). We did not find a significantly reduced C3b deposition on the two PorA(−) strains. Moreover, similar amounts of MAC were deposited on the two PorA(+) and the two PorA(−) variants, regardless of differences in serum sensitivity.
In an attempt to manipulate the bactericidal status of the serum, we added a chimeric anti-PorA monoclonal antibody (43) to the serum without bactericidal activity. Although the bactericidal activity of the anti-PorA antibody-supplemented serum raised significantly after addition of 1 μg of the anti-PorA antibody/ml, the MAC formation did not change. Therefore, our findings suggest that what is important for efficient meningococcal lysis is not the amount of MAC formed but a proper MAC insertion. In experiments with N. gonorrhoeae (23), multimeric C9 within the C5b-9 complex was bactericidal only in the presence of a specific bacterial antibody, indicating that such a bactericidal antibody may alter the site or the nature of attachment of C5b-9 to the bacterial surface, affecting MAC insertion by which transmembrane channels are formed. So, it seems that, as proposed for gonococci, bactericidal antibodies may affect the configuration or the site of insertion of the MAC into the surface of meningococci. Though the mechanism of complement activation on meningococci appears to be much more complex than that on gonococci, we believe that identification of such sites needs further investigation since antigens of such sites might be useful for vaccine design.
Formation of the complement components at the surface of meningococcal strains in our experiments occurred almost exclusively via the classical pathway. In the supernatants of the bacteria-challenged sera, however, increased amounts of C4d and of Bb were found, indicating activation of classical and alternative pathways, respectively. Thus, it appears that meningococci induce alternative complement activation taking place mostly in the fluid phase, distant from the bacterial surface. An explanation for such distant activation might be the release of outer cell wall blebs of the bacteria.
In conclusion, our results showed the following. (i) Similar amounts of C3b and its inactivated form were deposited on meningococcal surfaces in SBA+ and SBA− sera, irrespective of the presence of serum IgG and meningococcal surface PorA or LPS. (ii) MAC formation occurred even in the absence of IgG and in equal amounts on SBA− serum-sensitive and -resistant strains. (iii) The addition of an anti-PorA antibody to a SBA− serum did not increase MAC formation on a resistant strain while SBA against this strain evolved. These findings indicate that the key to meningococcal killing is probably a proper MAC insertion in the cell wall and not its quantity deposited on the surface.
Acknowledgments
This work was partly supported by INTAS, Brussels, grant 971-0108. We thank R Würzner for providing most of the anti-MAC antibodies and U. Vogel for critically reviewing the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Albertí, S., D. Álvarez, S. Merino, M. T. Casado, F. Vivanco, J. M. Tomás, and V. J. Benedí. 1996. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect. Immun. 64:4726-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, J., L. Berthelsen, B. Bech Jensen, and I. Lind. 1998. Dynamics of meningococcal carrier state and characterisation of the carrier strains: a longitudinal study within three cohorts of military recruits. Epidemiol. Infect. 121:85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi, S., and J. Tranum-Jensen. 1987. Damage to mammalian cells by proteins that form transmembrane pores. Rev. Physiol. Biochem. Pharmacol. 107:147-223. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi, S., R. Käflein, T. S. Halstensen, F. Hugo, K. T. Preissner, and T. E. Mollnes. 1988. Complement S-protein (vitronectin) is associated with cytolytic membrane-bound C5b-9 complexes. Clin. Exp. Immunol. 74:459-464. [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi, S., F. Hugo, and J. Tranum-Jensen. 1990. Functions and relevance of the terminal complement sequence. Blut 60:309-318. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright, K., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright, K. 1995. Meningococcal carriage and disease, p. 115-146. In K. Cartwright (ed.), Meningococcal disease, John Wiley and Sons Ltd., Chichester, United Kingdom.
- 8.Cartwright, K., R. Morris, H. Rumke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel vesicle vaccine containing multiple class 1 (PorA) outer-membrane proteins. Vaccine 17:2612-2619. [DOI] [PubMed] [Google Scholar]
- 9.de Kleijn, E. D., R. de Groot, J. Labadie, A. B. Lafeber, G. van den Dobbelsteen, L. van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. Rümke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 18:1456-1466. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa, J. E., and P. Densen. 1991. Infectious disease associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fijen, C. A. P., E. J. Kuijper, M. Drogari-Apiranthitou, Y. van Leeuwen, M. R. Daha, and J. Dankert. 1998. Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysaccharide vaccine. Clin. Exp. Immunol. 114:362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fijen, C. A. P., E. J. Kuijper, M. T. te Bulte, M. Daha, and J. Dankert. 1999. Assessment of complement deficient persons among patients with meningococcal disease in the Netherlands. Clin. Infect. Dis. 28:98-105. [DOI] [PubMed] [Google Scholar]
- 13.Finne, J., D. Bitter-Suermann, C. Goridis, and U. Finne. 1987. An IgG monoclonal antibody to group B meningococci cross-reacts with the developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J. Immunol. 138:4402-4407. [PubMed] [Google Scholar]
- 14.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-510. [DOI] [PubMed] [Google Scholar]
- 15.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon, D. L., J. Rice, J. J. Finlay-Jones, P. J. McDonald, and M. K. Hostetter. 1988. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J. Infect. Dis. 157:697-704. [DOI] [PubMed] [Google Scholar]
- 18.Harriman, G. R., E. R. Podack, A. I. Braude, L. C. Corbeil, A. F. Esser, and J. G. Curd. 1982. Activation of complement by serum-resistant Neisseria gonorrhoeae. Assembly of the membrane attack complex without subsequent cell death. J. Exp. Med. 156:1235-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis, G. A., and N. S. Vedros. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis, G. A. 1994. Alalysis of C3 deposition and degradation on Neisseria meningididis and Neisseria gonorrhoeae. Infect. Immun. 62:1755-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenne, D., and K. K. Stanley. 1985. Molecular cloning of S-protein, a link between complement, coagulation and cell-substrate adhesion. EMBO J. 4:3153-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joiner, K. A., C. H. Hammer, E. J. Brown, and M. M. Frank. 1982. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J. Exp. Med. 155:797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joiner, K. A., K. Warren, E. Brown, J. Swanson, and M. Frank. 1983. Studies on the mechanism of bacterial resistance to complement-mediated killing IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum resistant but not on serum-sensitive Neisseria gonorrhoeae. J. Immunol. 131:1443-1451. [PubMed] [Google Scholar]
- 24.Joiner, K. A., A. B. Tartanian, C. H. Hammer, and J. E. Schweinle. 1989. Multimeric C9 within C5b-9 deposits in unique locations in the cell wall of Salmonella typhimurium. J. Immunol. 142:4450-4457. [PubMed] [Google Scholar]
- 25.Jones, D. 1995. Epidemiology of meningococcal disease in Europe and the USA p. 147-158, In K. Cartwright (ed.), Meningococcal disease. John Wiley and Sons Ltd., Chichester, United Kingdom.
- 26.Kahler, C. M., L. E. Martin, G. C. Shih, M. M. Rahman, R. W. Carlson, and D. S. Stephens. 1998. The (α2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 66:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolb, W. P., P. R. Morrow, and J. D. Tamerius. 1989. Ba and Bb fragments of factor B activation: fragment production, biological activities, neoepitope expression and quantification in clinical samples. Complement Inflamm. 6:175-204. [DOI] [PubMed] [Google Scholar]
- 28.Loos, M., D. Bitter-Suermann, and M. Dierich. 1974. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial polysaccharides and with lipid A. J. Immunol. 112:935-940. [PubMed] [Google Scholar]
- 29.Loos, M., and F. Clas. 1987. Antibody independent killing of gram-negative bacteria via the classical pathway of complement. Immunol. Lett. 14:203-208. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon, F. G., R. Borrow, A. R. Gorringe, A. J. Fox, D. M. Jones, and A. Robinson. 1993. Demonstration of lipooligosaccharide immunotype and capsule as virulence factors for Neisseria meningitidis using an infant mouse intranasal infection model. Microb. Pathog. 15:359-366. [DOI] [PubMed] [Google Scholar]
- 31.Merino, S., S. Albertí, and J. M. Tomás. 1994. Aeromonas salmonicida resistance to complement-mediated killing. Infect. Immun. 62:5483-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer-membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mollnes, T. E., T. Lea, M. Harboe, and J. Tschopp. 1985. Monoclonal antibodies recognizing a neoantigen of poly(C9) detect the human terminal complement complex in tissue and plasma. Scand. J. Immunol. 22:183-195. [DOI] [PubMed] [Google Scholar]
- 34.Müller-Eberhard, H. J. 1975. Complement. Annu. Rev. Biochem. 44:97.. [DOI] [PubMed] [Google Scholar]
- 35.Peeters, C. C. A. M., H. C. Rümke, J. Meulenbelt, M. Schuller, A. J. Kuijpers, P. van der Ley, and J. T. Poolman. 1996. Phase I clinical trial with a hexavalent PorA containing meningococcal outer-membrane vesicle vaccine. Vaccine 14:1009-1015. [DOI] [PubMed] [Google Scholar]
- 36.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer-membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 37.Podack, E. R., and J. Tscopp. 1984. Membrane attack by complement. Mol. Immunol. 21:589-603. [DOI] [PubMed] [Google Scholar]
- 38.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, U. Vogel, M. Frosch, C. Elkins, H. K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B N. meningitidis. Mol. Immunol. 36:915-928. [DOI] [PubMed] [Google Scholar]
- 39.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer-membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross, S. G., and P. Densen. 1984. Complement deficiency states and infection. Medicine 63:243-273. [PubMed] [Google Scholar]
- 41.Steeghs, L., R. d Hartog, A. de Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 42.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 43.Van den Elsen, J., L. Vandeputte-Rutten, J. Kroon, and R. Gros. 1999. Bactericidal antibody recognition of meningococcal PorA by induced fit. J. Biol. Chem. 274:1495-1501. [DOI] [PubMed] [Google Scholar]
- 44.Van der Ende, A., C. T. P. Hopman, and J. Dankert. 1999. Deletion of porA by recombination between clusters of repetitive extragenic palindromic sequences in Neisseria meningitidis. Infect. Immun. 67:2928-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Ende, A., C. T. P. Hopman, and J. Dankert. 2000. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect. Immun. 68:6685-6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogel, U., S. Hammerschmidt, and M. Frosch. 1996. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitidis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med. Microbiol. Immunol. Berlin 185:81-87. [DOI] [PubMed] [Google Scholar]
- 47.Vogel, U., A. Weinberger, R. Frank, A. Mueller, J. Koehl, J. P. Atkinson, and M. Frosch. 1997. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect. Immun. 65:4022-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel, U., H. Claus, G. Heinze, and M. Frosch. 1997. Functional characterization of an isogenic meningococcal a-2,3-sialyltransferase mutant: the role of lipooligosaccharide for serum resistance in serogroup B meningococci. Med. Microbiol. Immunol. 186:159-166. [DOI] [PubMed] [Google Scholar]
- 49.Vogel, U., and M. Frosch. 1999. Mechanisms of neisserial serum resistance. Mol. Microb. 32:1133-1139. [DOI] [PubMed] [Google Scholar]
- 50.Wyle, F. A., M. S. Artenstein, B. L. Brandt, E. C. Traumont, D. L. Kasper, P. L. Alteri, S. L. Berman, and J. P. Lowenthal. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccine. J. Infect. Dis. 126:514-522. [DOI] [PubMed] [Google Scholar]