Abstract
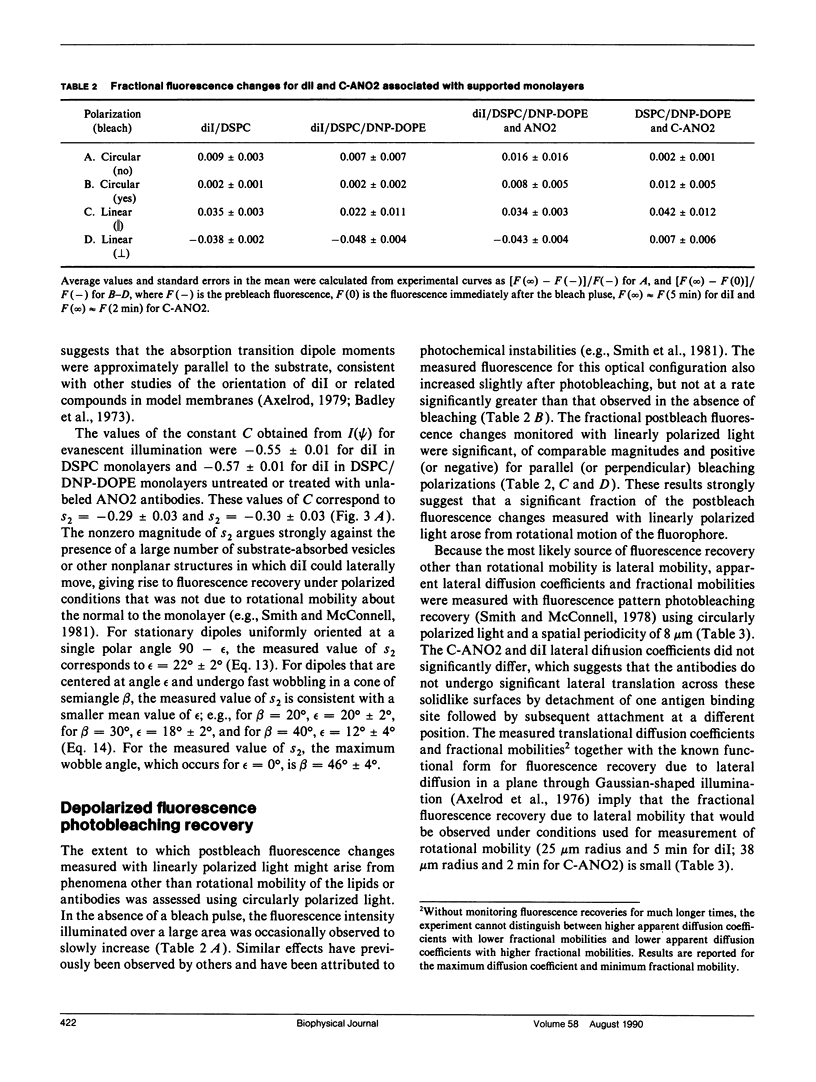
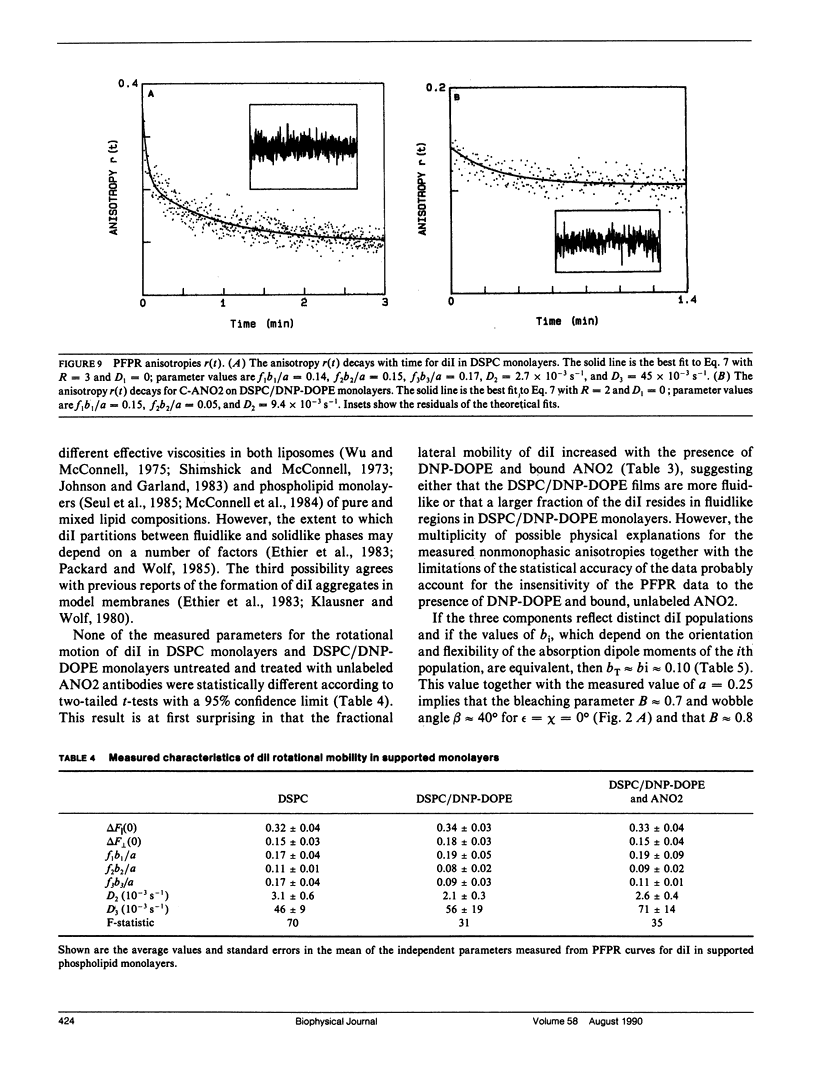
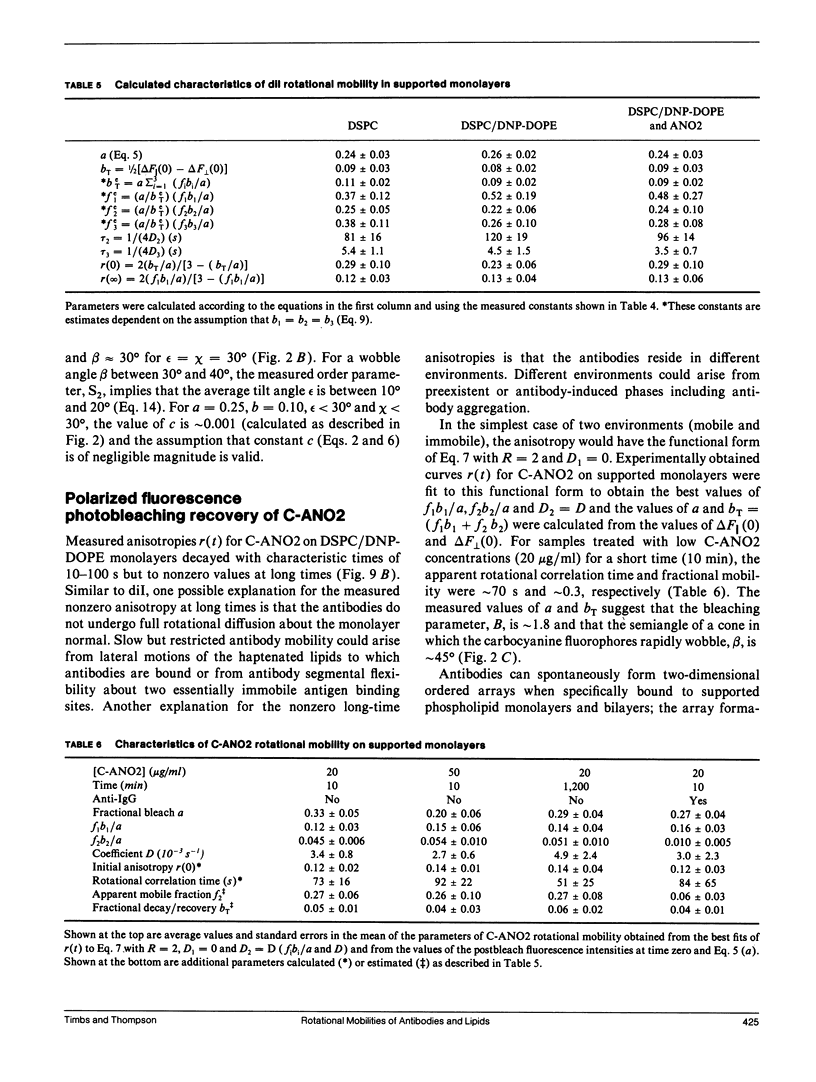
Polarized fluorescence photobleaching recovery has been used to monitor slow rotational motions of a fluorescently-labeled anti-dinitrophenyl mouse IgGl monoclonal antibody (ANO2) specifically bound to substrate-supported monolayers composed of a mixture of distearoylphosphatidylcholine (DSPC) and dinitrophenyldioleoylphosphatidylethanolamine (DNP-DOPE). ANO2 antibodies were labeled with a new bifunctional carbocyanine fluorophore that has two amino-reactive groups; steady-state fluorescence anisotropy data confirmed the expected result that the ANO2-conjugated bifunctional probe had less independent flexibility than ANO2-conjugated unifunctional fluorescence labels. Rotational mobilities were also measured for the fluorescent lipid 1,1'-dioctadecyl 3,3,3',3'-tetramethylindocarbocyanine (dil) in DSPC and in mixed DSPC/DNP-DOPE monolayers in the presence and absence of unlabeled ANO2 antibodies. The apparent rotational correlation time and fractional mobility of ANO2 on supported monolayers were approximately 70 and approximately 0.3 s, respectively. These measured parameters of rotational mobility did not depend on the ANO2 surface density or on kinetic factors, but addition of unlabeled polyclonal anti-(mouse IgG) antibodies significantly decreased the apparent mobile fraction. The measured fluorescence recovery curves for dil were consistent with two fluorophore populations with rotational correlation times of approximately 4 and approximately 100 s and a population of immobile fluorescent lipid. No difference in fluorescence recovery and decay curves was measured for dil in DSPC monolayers, DSPC/DNP-DOPE monolayers, and DSPC/DNP-DOPE monolayers treated with unlabeled ANO2 antibodies.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization. Biophys J. 1979 Jun;26(3):557–573. doi: 10.1016/S0006-3495(79)85271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Cell surface heating during fluorescence photobleaching recovery experiments. Biophys J. 1977 Apr;18(1):129–131. doi: 10.1016/S0006-3495(77)85601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badley R. A., Martin W. G., Schneider H. Dynamic behavior of fluorescent probes in lipid bilayer model membranes. Biochemistry. 1973 Jan 16;12(2):268–275. doi: 10.1021/bi00726a015. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K., Hsu F. J., Hafeman D. G., McConnell H. M. Monoclonal antibodies to a nitroxide lipid hapten. Biochim Biophys Acta. 1982 Sep 13;721(1):30–38. doi: 10.1016/0167-4889(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Burghardt T. P., Thompson N. L. Effect of planar dielectric interfaces on fluorescence emission and detection. Evanescent excitation with high-aperture collection. Biophys J. 1984 Dec;46(6):729–737. doi: 10.1016/S0006-3495(84)84071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. E. Interpretation of fluorescence photobleaching recovery experiments on oriented cell membranes. FEBS Lett. 1985 Nov 18;192(2):255–258. doi: 10.1016/0014-5793(85)80119-8. [DOI] [PubMed] [Google Scholar]
- Ethier M. F., Wolf D. E., Melchior D. L. Calorimetric investigation of the phase partitioning of the fluorescent carbocyanine probes in phosphatidylcholine bilayers. Biochemistry. 1983 Mar 1;22(5):1178–1182. doi: 10.1021/bi00274a029. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., von Tscharner V., McConnell H. M. Specific antibody-dependent interactions between macrophages and lipid haptens in planar lipid monolayers. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4552–4556. doi: 10.1073/pnas.78.7.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-wei S., McConnell H. Phase separations in phospholipd membranes. Biochemistry. 1975 Feb 25;14(4):847–854. doi: 10.1021/bi00675a032. [DOI] [PubMed] [Google Scholar]
- Johnson P., Garland P. B. Carbocyanine dyes used as fluorescent triplet probes for measuring slow rotational diffusion of lipids in membranes. FEBS Lett. 1983 Mar 21;153(2):391–394. doi: 10.1016/0014-5793(83)80650-4. [DOI] [PubMed] [Google Scholar]
- Kimura K., Nakanishi M., Ueda M., Ueno J., Nariuchi H., Furukawa S., Yasuda T. The effect of immunoglobulin G1 structure on macrophage binding to supported planar lipid monolayers. Immunology. 1986 Oct;59(2):235–238. [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Wolf D. E. Selectivity of fluorescent lipid analogues for lipid domains. Biochemistry. 1980 Dec 23;19(26):6199–6203. doi: 10.1021/bi00567a039. [DOI] [PubMed] [Google Scholar]
- Leahy D. J., Rule G. S., Whittaker M. M., McConnell H. M. Sequences of 12 monoclonal anti-dinitrophenyl spin-label antibodies for NMR studies. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3661–3665. doi: 10.1073/pnas.85.11.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M., Poo M. M. Protein diffusion in cell membranes: some biological implications. Int Rev Cytol. 1984;87:19–81. doi: 10.1016/s0074-7696(08)62439-0. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Tamm L. K., Weis R. M. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci U S A. 1984 May;81(10):3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Metzger H. Early molecular events in antigen-antibody cell activation. Annu Rev Pharmacol Toxicol. 1979;19:427–445. doi: 10.1146/annurev.pa.19.040179.002235. [DOI] [PubMed] [Google Scholar]
- Packard B. S., Wolf D. E. Fluorescence lifetimes of carbocyanine lipid analogues in phospholipid bilayers. Biochemistry. 1985 Sep 10;24(19):5176–5181. doi: 10.1021/bi00340a033. [DOI] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Thompson N. L. High-order fluorescence fluctuation analysis of model protein clusters. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6148–6152. doi: 10.1073/pnas.86.16.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Poglitsch C. L., Thompson N. L. Interaction of antibodies with Fc receptors in substrate-supported planar membranes measured by total internal reflection fluorescence microscopy. Biochemistry. 1990 Jan 9;29(1):248–254. doi: 10.1021/bi00453a033. [DOI] [PubMed] [Google Scholar]
- Scalettar B. A., Selvin P. R., Axelrod D., Hearst J. E., Klein M. P. A fluorescence photobleaching study of the microsecond reorientational motions of DNA. Biophys J. 1988 Feb;53(2):215–226. doi: 10.1016/S0006-3495(88)83083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalettar B. A., Selvin P. R., Axelrod D., Klein M. P., Hearst J. E. A polarized photobleaching study of DNA reorientation in agarose gels. Biochemistry. 1990 May 22;29(20):4790–4798. doi: 10.1021/bi00472a007. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., McConnell H. M., Smith Baron A., Parce J. W. Pattern photobleaching of fluorescent lipid vesicles using polarized laser light. Biophys J. 1981 Jan;33(1):139–146. doi: 10.1016/S0006-3495(81)84877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Parce J. W., Smith B. A., McConnell H. M. Antibodies bound to lipid haptens in model membranes diffuse as rapidly as the lipids themselves. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4177–4179. doi: 10.1073/pnas.76.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Weis R. M., McConnell H. M. Measurement of rotational motion in membranes using fluorescence recovery after photobleaching. Biophys J. 1981 Oct;36(1):73–91. doi: 10.1016/S0006-3495(81)84717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Seul M., McConnell H. M. Lateral diffusion of specific antibodies bound to lipid monolayers on alkylated substrates. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1169–1173. doi: 10.1073/pnas.83.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L. K. Lateral diffusion and fluorescence microscope studies on a monoclonal antibody specifically bound to supported phospholipid bilayers. Biochemistry. 1988 Mar 8;27(5):1450–1457. doi: 10.1021/bi00405a009. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., McConnell H. M., Burhardt T. P. Order in supported phospholipid monolayers detected by the dichroism of fluorescence excited with polarized evanescent illumination. Biophys J. 1984 Dec;46(6):739–747. doi: 10.1016/S0006-3495(84)84072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgiris E. E. Antibody crystallization on phospholipid films: dynamics and the effects of antibody conformation. J Cell Biochem. 1985;29(3):239–251. doi: 10.1002/jcb.240290308. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E., Kornberg R. D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen--antibody--complement complexes. Nature. 1983 Jan 13;301(5896):125–129. doi: 10.1038/301125a0. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E. Supported phospholipid bilayers for two-dimensional protein crystallization. Biochem Biophys Res Commun. 1986 Jan 29;134(2):819–826. doi: 10.1016/s0006-291x(86)80494-6. [DOI] [PubMed] [Google Scholar]
- Velez M., Axelrod D. Polarized fluorescence photobleaching recovery for measuring rotational diffusion in solutions and membranes. Biophys J. 1988 Apr;53(4):575–591. doi: 10.1016/S0006-3495(88)83137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. A. Fluorescence recovery spectroscopy as a probe of slow rotational motions. Biophys J. 1984 Dec;46(6):795–803. doi: 10.1016/S0006-3495(84)84078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. A., Rigler R. Separation of translational and rotational contributions in solution studies using fluorescence photobleaching recovery. Biophys J. 1984 Dec;46(6):787–793. doi: 10.1016/S0006-3495(84)84077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. L., Palmer A. G., 3rd, Thompson N. L. Inhomogeneous translational diffusion of monoclonal antibodies on phospholipid Langmuir-Blodgett films. Biophys J. 1988 Sep;54(3):463–470. doi: 10.1016/S0006-3495(88)82979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguerabide J., Stryer L. Fluorescence spectroscopy of an oriented model membrane. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1217–1221. doi: 10.1073/pnas.68.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V., McConnell H. M. Physical properties of lipid monolayers on alkylated planar glass surfaces. Biophys J. 1981 Nov;36(2):421–427. doi: 10.1016/S0006-3495(81)84741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


