Abstract
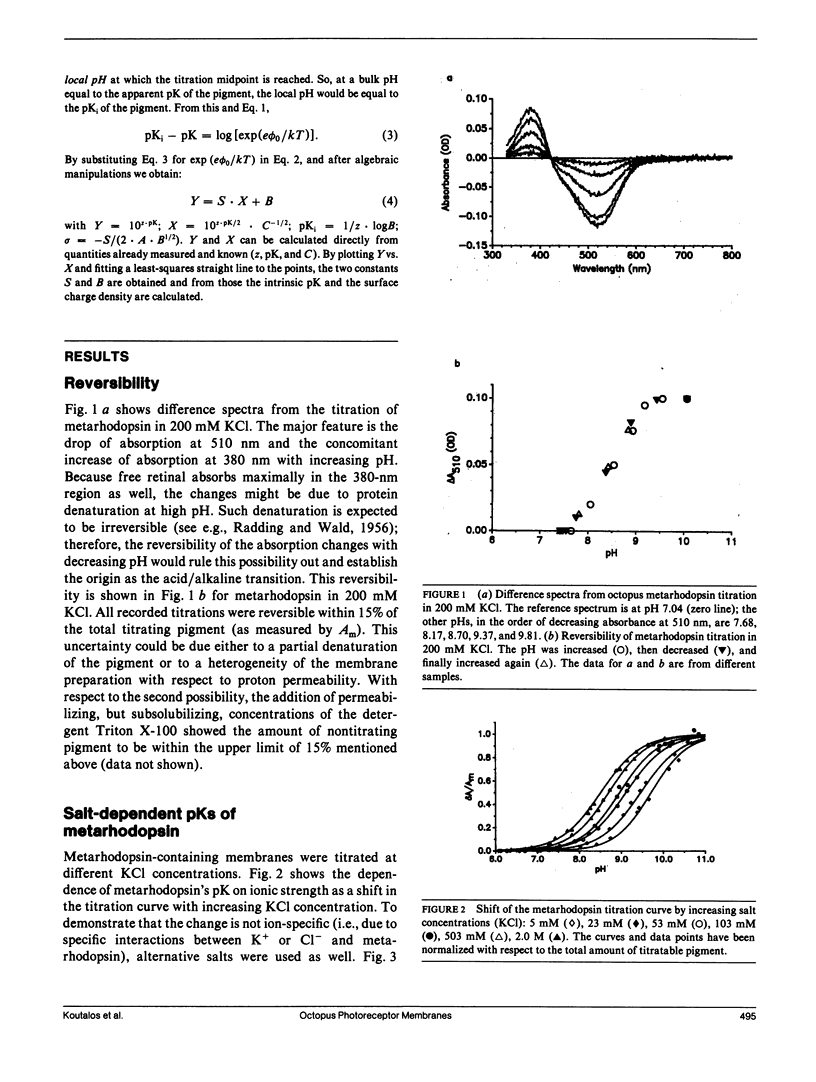
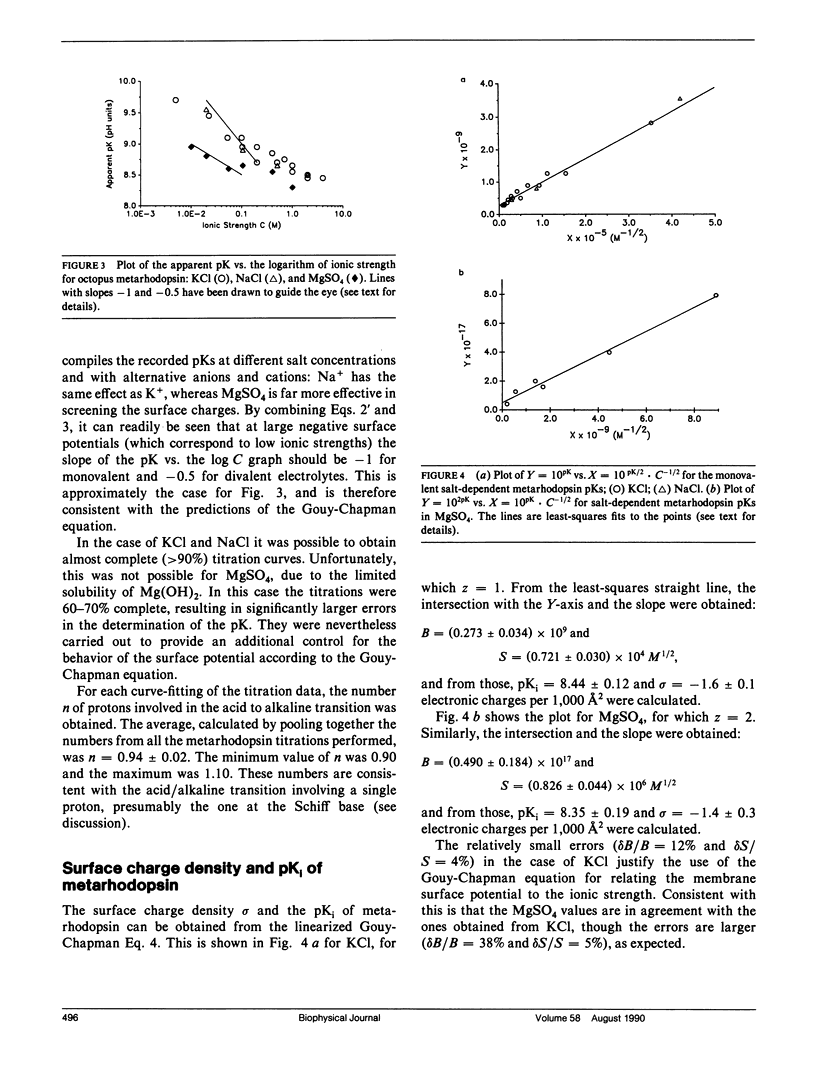
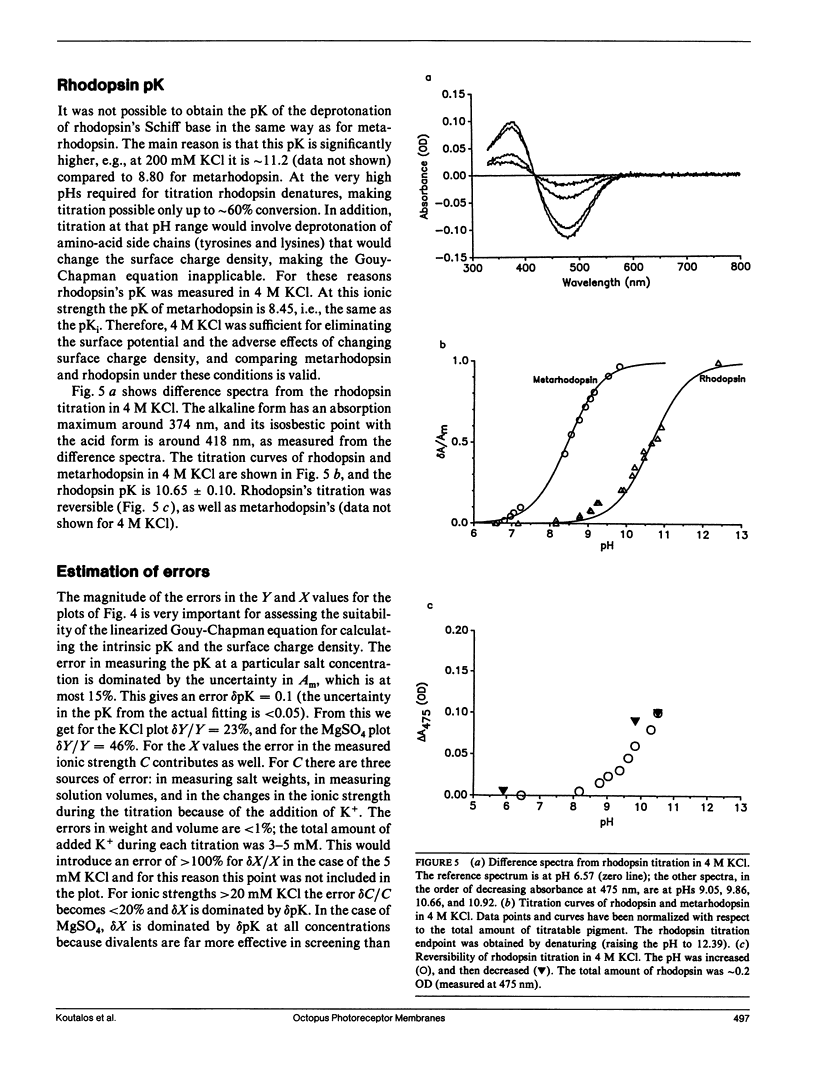
The chromophore of octopus rhodopsin is 11-cis retinal, linked via a protonated Schiff base to the protein backbone. Its stable photoproduct, metarhodopsin, has all-trans retinal as its chromphore. The Schiff base of acid metarhodopsin (lambda max = 510 nm) is protonated, whereas that of alkaline metarhodopsin (lambda max = 376 nm) is unprotonated. Metarhodopsin in photoreceptor membranes was titrated and the apparent pK of the Schiff base was measured at different ionic strengths. From these salt-dependent pKs the surface charge density of the octopus photoreceptor membranes and the intrinsic Schiff base pK of metarhodopsin were obtained. The surface charge density is sigma = -1.6 +/- 0.1 electronic charges per 1,000 A2. Comparison of the measured surface charge density with values from octopus rhodopsin model structures suggests that the measured value is for the extracellular surface and so the Schiff base in metarhodopsin is freely accessible to protons from the extracellular side of the membrane. The intrinsic Schiff base pK of metarhodopsin is 8.44 +/- 0.12, whereas that of rhodopsin is found to be 10.65 +/- 0.10 in 4.0 M KCl. These pK values are significantly higher than the pK value around 7.0 for a retinal Schiff base in a polar solvent; we suggest that a plausible mechanism to increase the pK of the retinal pigments is the preorganization of their chromophore-binding sites. The preorganized site stabilizes the protonated Schiff base with respect to the unprotonated one. The difference in the pK for the octopus rhodopsin compared with metarhodopsin is attributed to the relative freedom of the latter's chromophore-binding site to rearrange itself after deprotonation of the Schiff base.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akino T., Tsuda M. Characteristics of phospholipids in microvillar membranes of octopus photoreceptor cells. Biochim Biophys Acta. 1979 Sep 4;556(1):61–71. doi: 10.1016/0005-2736(79)90419-x. [DOI] [PubMed] [Google Scholar]
- Baasov T., Friedman N., Sheves M. Factors affecting the C = N stretching in protonated retinal Schiff base: a model study for bacteriorhodopsin and visual pigments. Biochemistry. 1987 Jun 2;26(11):3210–3217. doi: 10.1021/bi00385a041. [DOI] [PubMed] [Google Scholar]
- Birge R. R., Einterz C. M., Knapp H. M., Murray L. P. The nature of the primary photochemical events in rhodopsin and isorhodopsin. Biophys J. 1988 Mar;53(3):367–385. doi: 10.1016/S0006-3495(88)83114-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A., Dixon S. F., Tsuda M. Photoenergetics of octopus rhodopsin. Convergent evolution of biological photon counters? Eur Biophys J. 1986;13(4):195–201. doi: 10.1007/BF00260367. [DOI] [PubMed] [Google Scholar]
- Deng H., Callender R. H. A study of the Schiff base mode in bovine rhodopsin and bathorhodopsin. Biochemistry. 1987 Nov 17;26(23):7418–7426. doi: 10.1021/bi00397a033. [DOI] [PubMed] [Google Scholar]
- Doukas A. G., Aton B., Callender R. H., Ebrey T. G. Resonance Raman studies of bovine metarhodopsin I and metarhodopsin II. Biochemistry. 1978 Jun 13;17(12):2430–2435. doi: 10.1021/bi00605a028. [DOI] [PubMed] [Google Scholar]
- HUBBARD R., ST GEORGE R. C. The rhodopsin system of the squid. J Gen Physiol. 1958 Jan 20;41(3):501–528. doi: 10.1085/jgp.41.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L. Transbilayer coupling mechanism for the formation of lipid asymmetry in biological membranes. Application to the photoreceptor disc membrane. Biophys J. 1990 Jan;57(1):99–108. doi: 10.1016/S0006-3495(90)82510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T., Tsuda M. Resonance Raman spectra of octopus acid and alkaline metarhodopsins. Biochim Biophys Acta. 1980 Jul 24;624(1):211–217. doi: 10.1016/0005-2795(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Koutalos Y., Ebrey T. G. Recent progress in vertebrate photoreception. Photochem Photobiol. 1986 Dec;44(6):809–817. doi: 10.1111/j.1751-1097.1986.tb05541.x. [DOI] [PubMed] [Google Scholar]
- Koutalos Y., Ebrey T. G., Tsuda M., Odashima K., Lien T., Park M. H., Shimizu N., Derguini F., Nakanishi K., Gilson H. R. Regeneration of bovine and octopus opsins in situ with natural and artificial retinals. Biochemistry. 1989 Mar 21;28(6):2732–2739. doi: 10.1021/bi00432a055. [DOI] [PubMed] [Google Scholar]
- Lisman J. E., Schulman S., Sheline Y., Brown P. K. Properties of the pH-sensitive site that controls the lambda max of Limulus metarhodopsin. J Gen Physiol. 1981 Feb;77(2):191–203. doi: 10.1085/jgp.77.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Nashima K., Kawase N., Kito Y. Effect of phospholipid and detergent on the Schiff base of cephalopod rhodopsin and metarhodopsin. Biochim Biophys Acta. 1980 Dec 16;626(2):390–396. doi: 10.1016/0005-2795(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Zolotarev A. S., Artamonov I. D., Bespalov I. A., Dergachev A. E., Tsuda M. Octopus rhodopsin. Amino acid sequence deduced from cDNA. FEBS Lett. 1988 May 9;232(1):69–72. doi: 10.1016/0014-5793(88)80388-0. [DOI] [PubMed] [Google Scholar]
- Pande C., Pande A., Yue K. T., Callender R., Ebrey T. G., Tsuda M. Resonance Raman spectroscopy of octopus rhodopsin and its photoproducts. Biochemistry. 1987 Aug 11;26(16):4941–4947. doi: 10.1021/bi00390a009. [DOI] [PubMed] [Google Scholar]
- Parkes J. H., Liebman P. A. Temperature and pH dependence of the metarhodopsin I-metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions. Biochemistry. 1984 Oct 9;23(21):5054–5061. doi: 10.1021/bi00316a035. [DOI] [PubMed] [Google Scholar]
- Paulsen R., Zinkler D., Delmelle M. Architecture and dynamics of microvillar photoreceptor membranes of a cephalopod. Exp Eye Res. 1983 Jan;36(1):47–56. doi: 10.1016/0014-4835(83)90088-x. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Cobbs W. H. Visual transduction in vertebrate rods and cones: a tale of two transmitters, calcium and cyclic GMP. Vision Res. 1986;26(10):1613–1643. doi: 10.1016/0042-6989(86)90051-9. [DOI] [PubMed] [Google Scholar]
- RADDING C. M., WALD G. The stability of rhodopsin and opsin; effects of pH and aging. J Gen Physiol. 1956 Jul 20;39(6):923–933. doi: 10.1085/jgp.39.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheves M., Albeck A., Friedman N., Ottolenghi M. Controlling the pKa of the bacteriorhodopsin Schiff base by use of artificial retinal analogues. Proc Natl Acad Sci U S A. 1986 May;83(10):3262–3266. doi: 10.1073/pnas.83.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., McCain D. A., Nakanishi K., Okabe M., Shimizu N., Rodman H., Honig B., Bogomolni R. A. Chromophore/protein interaction in bacterial sensory rhodopsin and bacteriorhodopsin. Biophys J. 1986 Feb;49(2):479–483. doi: 10.1016/S0006-3495(86)83657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Zhukovsky E. A., Oprian D. D. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989 Nov 17;246(4932):928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- de Groot H. J., Harbison G. S., Herzfeld J., Griffin R. G. Nuclear magnetic resonance study of the Schiff base in bacteriorhodopsin: counterion effects on the 15N shift anisotropy. Biochemistry. 1989 Apr 18;28(8):3346–3353. doi: 10.1021/bi00434a033. [DOI] [PubMed] [Google Scholar]


