Abstract
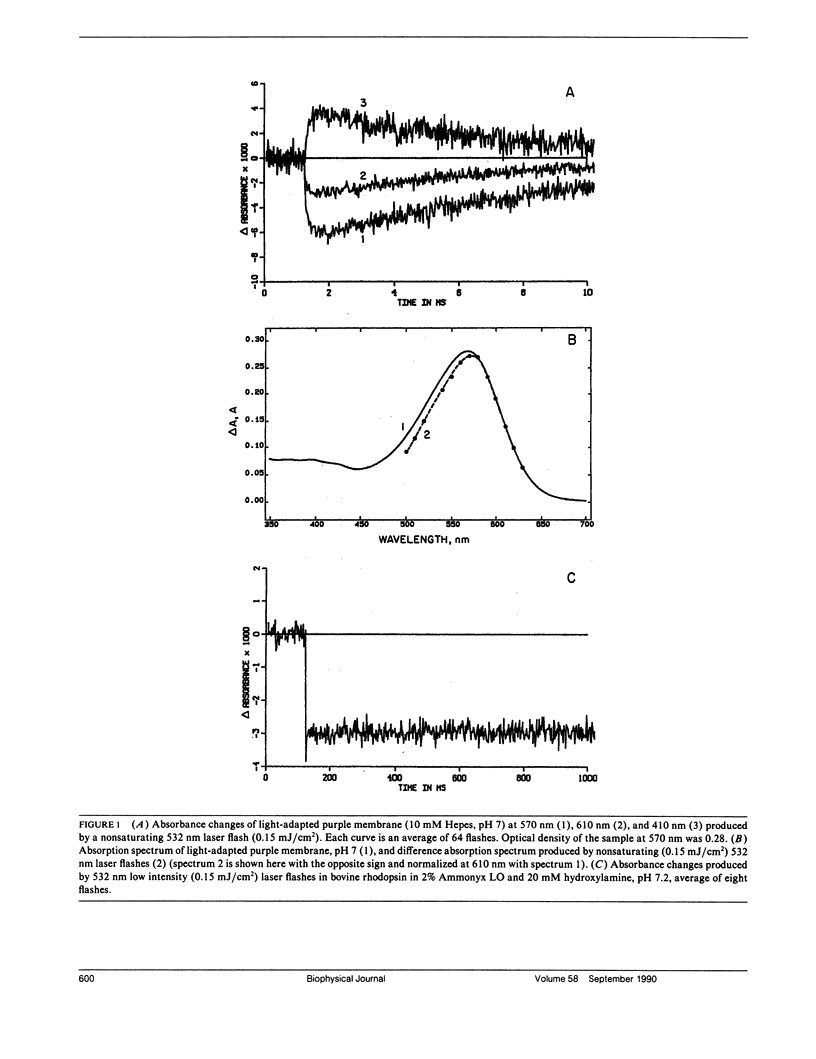
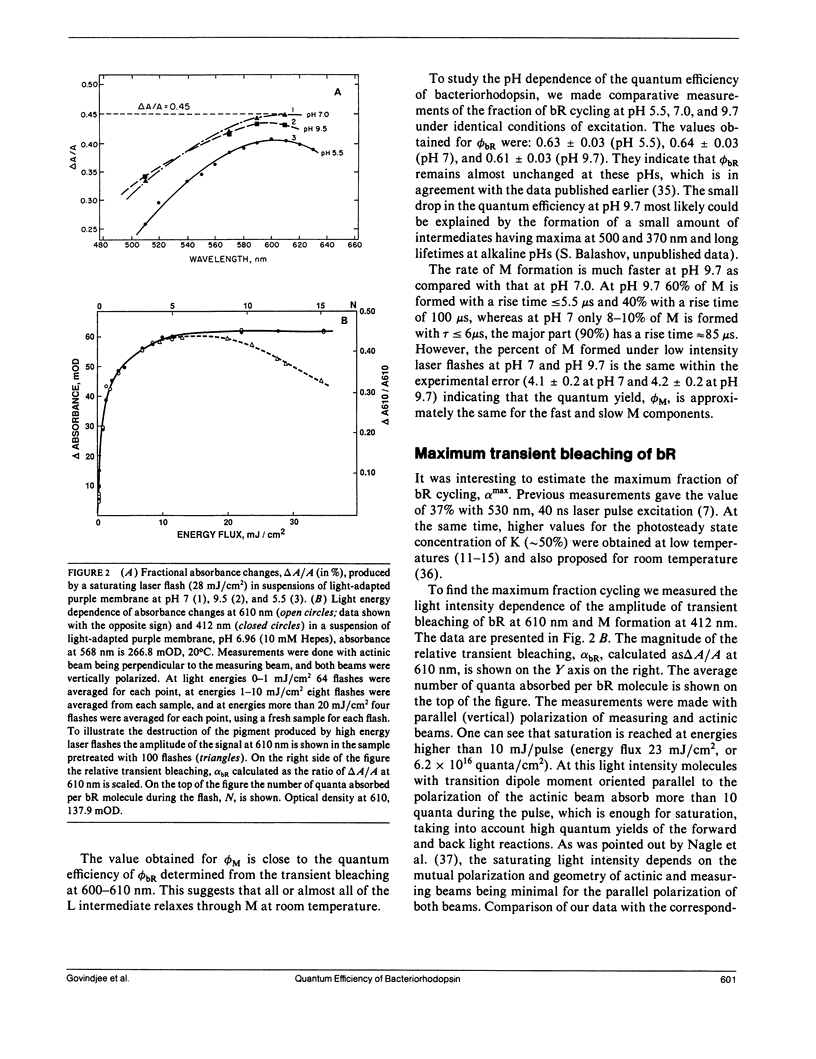
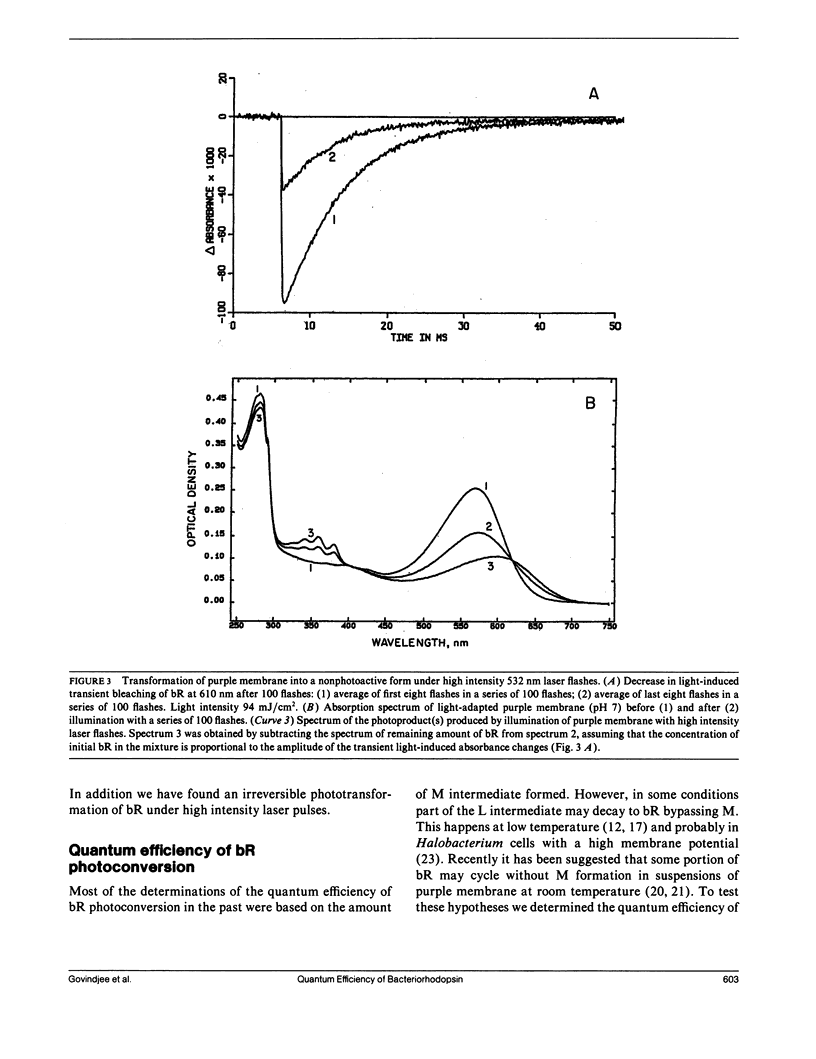
Values in the literature for the quantum efficiency of the photochemical cycle of bacteriorhodopsin (bR) range from 0.25 to 0.79 and the sum of the quantum yields of the forward and back photoreactions [Formula: see text] has been proposed to be 1. In the present work, low intensity laser flashes (532 nm) and kinetic spectroscopy were used to determine the quantum efficiency of bR photoconversion, [UNK]bR, by measuring transient bleaching of bR at 610 nm in the millisecond time scale. Bovine rhodopsin (R) in 2% ammonyx LO was used as a photon counter. We find that the ratio of the quantum yields of bacteriorhodopsin photoconversion and bleaching of rhodopsin, [UNK]bR/[UNK]R, is 0.96 ± 0.04. Based on the quantum yield of the photobleaching of rhodopsin, 0.67, the quantum efficiency of bR photoconversion was determined to be 0.64 ± 0.04. The quantum yield of M formation was found to be 0.65 ± 0.06. From the transient bleaching of bR at 610 nm with a saturating laser flash (28 mJ/cm2) the maximum amount of bR cycling was estimated to be 47 ± 3%. From this value and the spectrum of K published in the literature, the ratio of the efficiencies of the forward and back light reactions, [UNK]1/[UNK]2, was estimated to be 0.67 ± 0.06 and so [UNK]2 ≈ 1 (0.94 ± 0.06). The sum of [UNK]1 + [UNK]2 ≈ 1.6. It was found that repeated high-intensity laser flashes (>20 mJ/cm2) irreversibly transformed bR into two stable photoproducts. One has its absorption maximum at 605 nm and the other has a well-resolved vibronic spectrum with maxima at 342, 359 (main peak), and 379 nm. The quantum yield of the formation of the photoproducts is ≈ 10-4.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. The quantum efficiency for the photochemical conversion of the purple membrane protein. Biophys J. 1977 Feb;17(2):185–191. doi: 10.1016/S0006-3495(77)85636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Action spectrum and quantum efficiency for proton pumping in Halobacterium halobium. Biochemistry. 1980 May 13;19(10):2152–2159. doi: 10.1021/bi00551a024. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancsházy Z., Govindjee R., Ebrey T. G. Independent photocycles of the spectrally distinct forms of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6358–6361. doi: 10.1073/pnas.85.17.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C. R., Kalisky O., Rosenfeld T., Ottolenghi M. The quantum efficiency of the bacteriorhodopsin photocycle. Biophys J. 1977 Feb;17(2):179–183. doi: 10.1016/S0006-3495(77)85635-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C. R., Ottolenghi M., Korenstein R. On the primary quantum yields in the bacteriorhodopsin photocycle. Biophys J. 1976 Jul;16(7):839–843. doi: 10.1016/S0006-3495(76)85732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Ebrey T. G., Crofts A. R. The quantum efficiency of proton pumping by the purple membrane of Halobacterium halobium. Biophys J. 1980 May;30(2):231–242. doi: 10.1016/S0006-3495(80)85091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Lozier R. H. Photocycles of bacteriorhodopsin in light- and dark-adapted purple membrane studied by time-resolved absorption spectroscopy. Biophys J. 1989 Oct;56(4):693–706. doi: 10.1016/S0006-3495(89)82716-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G. Energy transfer in the purple membrane of Halobacterium halobium. Biophys J. 1978 Apr;22(1):49–66. doi: 10.1016/S0006-3495(78)85470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A., Stoeckenius W. Salt and pH-dependent changes of the purple membrane absorption spectrum. Photochem Photobiol. 1984 Nov;40(5):641–646. doi: 10.1111/j.1751-1097.1984.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I., Hwang S. B. Proton transport by bacteriorhodopsin in planar membranes assembled from air-water interface films. J Gen Physiol. 1980 Dec;76(6):649–682. doi: 10.1085/jgp.76.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W. The photochemical cycle of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1805–1809. [PubMed] [Google Scholar]
- Marinetti T. Nonproton ion release by purple membranes exhibits cooperativity as shown by determination of the optical cross-section. Biophys J. 1988 Aug;54(2):197–204. doi: 10.1016/S0006-3495(88)82948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R. A., Brito Cruz C. H., Pollard W. T., Shank C. V. Direct observation of the femtosecond excited-state cis-trans isomerization in bacteriorhodopsin. Science. 1988 May 6;240(4853):777–779. doi: 10.1126/science.3363359. [DOI] [PubMed] [Google Scholar]
- Milder S. J., Kliger D. S. A time-resolved spectral study of the K and KL intermediates of bacteriorhodopsin. Biophys J. 1988 Mar;53(3):465–468. doi: 10.1016/S0006-3495(88)83124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Parodi L. A., Lozier R. H. Procedure for testing kinetic models of the photocycle of bacteriorhodopsin. Biophys J. 1982 May;38(2):161–174. doi: 10.1016/S0006-3495(82)84543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hegemann P., Tittor J. The photocycle of the chloride pump halorhodopsin. II: Quantum yields and a kinetic model. EMBO J. 1985 Sep;4(9):2351–2356. doi: 10.1002/j.1460-2075.1985.tb03938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Schuhmann L. Reconstitution of bacteriorhodopsin. FEBS Lett. 1974 Aug 30;44(3):262–265. doi: 10.1016/0014-5793(74)81153-1. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polland H. J., Franz M. A., Zinth W., Kaiser W., Kölling E., Oesterhelt D. Early picosecond events in the photocycle of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):651–662. doi: 10.1016/S0006-3495(86)83692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M., Delmelle M. The photochemical quantum yield of bacteriorhodopsin is pH independent: a photoacoustic study. FEBS Lett. 1981 Jun 15;128(2):245–248. doi: 10.1016/0014-5793(81)80091-9. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Braiman M. S., Mogi T., Stern L. J., Khorana H. G. Conserved amino acids in F-helix of bacteriorhodopsin form part of a retinal binding pocket. FEBS Lett. 1989 Jul 3;250(2):448–452. doi: 10.1016/0014-5793(89)80774-4. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Keszthelyi L., Lanyi J. K. Transient spectroscopy of bacterial rhodopsins with an optical multichannel analyzer. 1. Comparison of the photocycles of bacteriorhodopsin and halorhodopsin. Biochemistry. 1989 Jun 13;28(12):5165–5172. doi: 10.1021/bi00438a038. [DOI] [PubMed] [Google Scholar]


