Abstract
Insertion mutations were isolated in cya and crp of Yersinia enterocolitica, which encode adenylate cyclase and the cyclic AMP (cAMP) receptor protein (CRP). The cya and crp mutants were affected for the production of proteins exported by the Ysc, Ysa, and flagellar type III secretion systems (TTSS). Protein production by each TTSS was restored when the respective mutation was complemented by a plasmid-encoded copy of the wild-type gene. Both cya and crp mutants exhibited reduced virulence for orally infected BALB/c mice in a 50% lethal dose analysis. Examination of bacterial survival in host tissues showed that cya and crp mutants colonized Peyer's patches and, to a lesser extent, mesenteric lymph nodes. However, the mutants did not appear to disseminate to the liver and spleen of infected mice. An initial examination of the effectiveness of Y. enterocolitica cya and crp mutants to stimulate protective immunity against subsequent challenge with virulent bacteria in mice was promising. The results indicate that the cAMP-CRP regulatory system is required for Y. enterocolitica virulence.
Yersinia enterocolitica is a gram-negative pathogen that causes gastrointestinal illnesses in humans and other animals. This bacterium is closely related to two other pathogenic species, Yersinia pseudotuberculosis, which also can cause gastrointestinal illness, and Yersinia pestis, which causes bubonic and pneumonic plague syndromes (7). Many chromosomal and plasmid-encoded genes that are conserved in Y. pseudotuberculosis and Y. pestis have been shown to contribute to Y. enterocolitica virulence (4). Control of virulence gene expression is influenced by a variety of environmental stimuli, and proper coordination of genes is likely to require the functions of specific and global regulatory systems (15, 29, 31).
Pathogenic species of Yersinia secrete many proteins that have been shown or postulated to influence pathogenesis. Three different pathways for extracellular protein secretion that are believed to transport proteins by a type III mechanism have been described for Y. enterocolitica (8, 13, 34). Each pathway responds differently to a number of environmental stimuli when examined in the laboratory, but it has been proposed that all are induced during infection. The most intensively studied protein secretion system common to all pathogenic yersiniae, among the type III secretion systems (TTSS), is that of Ysc (8). This system has been shown to be required for the export of a set of proteins referred to as Yops (Yersinia outer proteins). Under specific laboratory conditions, protein secretion by the Ysc TTSS is induced by growth at 37°C in a medium limited for calcium. However, when secretion is studied in the context of cell-based assays of virulence, the Ysc TTSS functions to directly inject Yop proteins into eukaryotic cells and affects a number of cellular activities (6, 17, 30). Several studies have shown that the function of specific Yops and the Ysc TTSS is necessary for virulence (8).
A second TTSS was recently identified in Y. enterocolitica; it is referred to as the Ysa TTSS (13). This system is induced in vitro at 26°C in a high-salt medium and is required for the secretion of a set of proteins called Ysps (Yersinia secreted proteins). The function of individual Ysp proteins has not been determined, but the Ysa TTSS is required for full virulence (13). A third secretion system that targets proteins by a type III mechanism is an integral part of the Y. enterocolitica flagellum. The flagellar TTSS has been shown to be required for the export of flagellar outer proteins (Fops) (34). One of the Fops is a phospholipase (YplA) that is required for virulence (25, 34). Similar to the case for the Ysa TTSS, under laboratory conditions, protein secretion by the flagellar TTSS occurs at 26°C but is maximally induced in a low-salt medium (26, 34).
The Ysc, Ysa, and flagellar TTSS have been implicated in Y. enterocolitica virulence, suggesting that each system is expressed at some point during the infectious cycle. While different in vitro conditions have been identified to induce optimal expression of these systems, the possibility remained that expression of genes necessary for the function of these TTSS requires common regulatory factors. In this study, evidence is presented suggesting that the ubiquitous signaling molecule cyclic AMP (cAMP) and the cAMP receptor protein (CRP) are necessary for normal expression of the Ysc, Ysa, and flagellar TTSS and for Y. enterocolitica virulence.
MATERIALS AND METHODS
Bacterial strains, plasmids and media.
The bacterial strains and plasmids used in this study are described in Table 1. Strains of Escherichia coli were routinely grown at 37°C, and Y. enterocolitica strains were grown at 26°C in Luria broth (1% tryptone, 0.5% yeast extract, and 90 mM NaCl) or on Luria agar (Difco). Media used for the examination of protein secretion by Y. enterocolitica were Luria broth base (L medium, 1% tryptone, and 0.5% yeast extract). Phospholipase indicator agar (PLA) consisted of L medium supplemented with 1% Tween 80 and 1 mM CaCl2 (34) and solidified with 1.5% (wt/vol) agarose (Difco). Antibiotics were used at the following concentrations: chloramphenicol (25 μg/ml for E. coli and 12.5 μg/ml for Y. enterocolitica), kanamycin (50 μg/ml), nalidixic acid (20 μg/ml), streptomycin (30 μg/ml), spectinomycin (30 μg/ml), and tetracycline (15 μg/ml for E. coli and 7.5 μg/ml for Y. enterocolitica).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Y. enterocolitica | ||
| JB580v | Serogroup O:8, Nalr, ΔyenR (R−, M+) | 16 |
| GY460 | ΔflhDC | 35 |
| GY455 | crp::TnMod-RKm′ | This study |
| GY562 | crp::TnMod-RKm′ | This study |
| GY572 | cya::TnMod-RKm′ | This study |
| GY654 | cya::TnMod-RKm′ | This study |
| YEDS10 | yplA-lacZY (pFUSE), yplA+ | 26 |
| GY4455 | yscK::TnMod-lacZYA-RKm′ | This study |
| GY4641 | ysaE::TnMod-lacZYA-RKm′ | K. Venecia and G. M. Younga |
| GY4531 | crp::str | This study |
| GY4651 | yscK::TnMod-lacZYA-RKm′, crp::str | This study |
| GY4659 | ysaE::TnMod-lacZYA-RKm′, crp::str | This study |
| GY843 | flhB-lacZYA (pFUSE), flhB+ | This study |
| GY4681 | flhB-lacZYA (pFUSE), flhB+, crp::str | This study |
| E. coli | ||
| S17-1 λpir | recA thi pro hsd (R− M+) RP4::2-Tc::Mu::Km Tn7 λpir | 27 |
| Plasmids | ||
| pGY355 | cya cloned into the EcoRI site of pTM100, Tcr | This study |
| pGY345 | crp cloned into the EcoRI site of pTM100, Tcr | This study |
| pGY362 | cya PCR product cloned into pCR-Blunt II-TOPO, Kmr | This study |
| pGY426 | crp PCR product cloned into pCR-Blunt II-TOPO, Kmr | This study |
| pGY364 | crp cloned into the suicide plasmid pEP185.2 | This study |
| pGY365 | crp::str cloned into the suicide plasmid pEP185.2 | This study |
| pEP185.2 | mob+, oriR6K, Cmr | 16 |
| pTM100 | mob+, derivative of pACYC184, Cmr Tcr | 19 |
| pCR-Blunt II-TOPO | Kmr | Invitrogen |
| pTnMod-RKm′ | TnMod-RKm′ in a delivery vector | 11 |
| pTnMod-lacZYA-RKm′ | pTnMod-RKm′ containing a promoterless lacZYA | Andrew Darwin |
| p34S-Sm | Plasmid containing a streptomycin resistance cassette | 11 |
Unpublished data.
Evaluation of motility.
Bacterial motility was directly examined by phase-contrast microscopy. Motility was also examined by inoculating bacteria at the center of a petri plate containing 1% (wt/vol) tryptone solidified with 0.35% agar (Difco) as described earlier (35). Motility was scored as positive if the strains exhibited growth and migration emanating from the point of inoculation.
Preparation of extracellular proteins and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Extracellular proteins were prepared as previously described (34). Y. enterocolitica was grown overnight in Luria broth and subcultured to an optical density at 600 nm (OD600) of 0.2 in 5 ml of appropriate media to induce secretion of Fops, Ysps, or Yops. A Fop is defined as protein that is secreted into the culture supernatant under conditions that induce the production of flagella, and its production is controlled by flagellar master regulators (34). To induce the flagellar TTSS, overnight cultures of selected strains were subcultured into L medium and grown at 26°C for 6 h with mild aeration. A Ysp is defined as a protein secreted into culture supernatants under conditions that induce the Ysa TTSS (13). Induction of the Ysa TTSS was achieved by subculturing strains into L medium supplemented with 300 mM NaCl, followed by growth at 26°C for 6 h with mild aeration. A Yop is a protein that is secreted into culture supernatants under conditions that induce the Ysc TTSS (20). The Ysc TTSS was induced by subculturing into L medium depleted of calcium by the addition of 20 mM sodium oxalate and 20 mM MgCl2. Cultures were grown for 2 h at 26°C and were then shifted to 37°C for 4 h.
To examine secreted proteins, cultures grown under appropriate inducing conditions were collected. The OD600 of the culture was determined at the time of harvesting. Bacterial cells were removed by centrifugation in a microcentrifuge at 8,000 × g for 5 min. The upper two-thirds of the supernatant was removed and centrifuged again. The upper two-thirds of the supernatant was then removed and passed through a nonpyrogenic 0.22-μm-pore-size filter (Gelman Inc.). Proteins were precipitated with 10% (wt/vol) ice-cold trichloroacetic acid and washed with ice-cold acetone. Proteins were suspended in sample buffer containing 2-mercaptoethanol (18), and the volume was adjusted according to the OD600 of the culture. Equivalent amounts of sample were analyzed by SDS-PAGE. Samples were heated to 95°C for 5 min and were then loaded onto 10% or 12.5% polyacrylamide gels. Proteins were visualized by staining with silver or Coomassie brilliant blue as indicated (2, 18).
Transposon mutagenesis.
Y. enterocolitica JB580v was mutagenized by conjugation of the plasmid pTnMod-RKm′ (11) using conditions described earlier (10). Forty separate matings were collected and plated on PLA containing nalidixic acid and kanamycin. Approximately 40,000 colonies were examined to identify strains that exhibited a phospholipase-negative phenotype that correlated with defects in YplA export (34). Colonies unaffected for YplA secretion had a halo of precipitation, while candidate mutants formed colonies that lacked a halo of precipitation.
Characterization of transposon insertions sites and DNA sequencing.
Chromosomal DNA was isolated from mutants that contained TnMod-RKm′ insertions and was digested with EcoRI. The digested DNA was ligated overnight, and replicating plasmids were recovered by electroporation of E. coli S17-1 λpir followed by selection for kanamycin resistance (11). Direct cloning of the transposon/chromosome junctions was facilitated by the presence of a conditional oriR6K in TnMod-RKm′ that can function in specialized E. coli strains that carry a copy of pir (27). Plasmids were isolated and analyzed by restriction digest to confirm the integrity of the transposon sequences. The sequence of the chromosomal DNA immediately adjacent to the transposon was then determined using primers that annealed near the ends of TnMod-RKm′ (primer KM1, 5′-CCCCGAGCTCTTAATTAA-3′, and primer KM2, 5′-GAACACTTAACGGCTGAC-3′). DNA sequence was obtained using an Applied Biosystems DNA sequencing system and the BigDye terminator cycle sequencing kit (Biosystems) according to the manufacturer's instructions.
Cloning of crp and cya from Y. enterocolitica.
The chromosomal region containing crp was amplified by PCR with Pfu polymerase (Stratagene) using oligonucleotide primers GYcrp1, 5′-CGG AAT TCC GGA CAC TAG CCT GTG C-3′, and GYcrp2, 5′-CGG AAT TCC GGT TTA TAG GGA ATT AAC G-3′, which corresponded to sequences predicted to encode crp of Y. pestis (21). The template DNA for the reaction was purified genomic DNA originating from Y. enterocolitica JB580v. The fragment of DNA generated by PCR was cloned into plasmid pCR-Blunt II-TOPO using the manufacturer's procedures (Invitrogen) to create pGY426. Subsequently a ca. 90-bp EcoRI fragment containing crp was subcloned into a corresponding EcoRI site of pTM100 generating pGY345.
The chromosomal region of cya was cloned by complementation. A cosmid library of Y. enterocolitica JB580v was introduced into Y. enterocolitica GY572 essentially as described earlier (35). Recipient strains were screened for the restoration of YplA activity on PLA indicator medium. Cosmids that restored YplA activity were identified, and one was used as a template to amplify the cya region using oligonucleotide primers (GYcya1, 5′-GCT TGC CAT AAG GCG AG-3′, and GYcya2, 5′-TAC ATC AGG TAT GGC-3′) based on the sequence of cya from Yersinia intermedia (32). DNA amplification, subsequent cloning, and subcloning were conducted by procedures described above for crp.
The DNA sequence of Y. enterocolitica crp and cya was determined using the BigDye terminator cycle sequencing kit (Biosystems) according to the manufacturer's instructions. Oligonucleotide primers were synthesized as necessary to generate sequence of both strands of each cloned DNA fragment. The sequences were compared to known DNA sequences in publicly accessible databases using the BLAST program available from the National Center for Biotechnology Information. The assembled crp and cya DNA sequences have been submitted to GenBank with accession numbers AY064698 and AY064699.
Construction of crp mutant strains.
Construction of strains containing the crp::str allele was done by allelic exchange as described earlier (22) using plasmid pGY365. Plasmid pGY365 was constructed by subcloning a ca. 900-bp EcoRI fragment containing crp from pGY426 into a unique EcoRV site of the suicide plasmid pEP185.2 to create pGY364. The EcoRI fragment was treated with the Klenow fragment of DNA polymerase to create blunt ends prior to DNA ligation. This cloning step destroyed the EcoRV site of the cloning vector. Plasmid pGY364 was then digested with EcoRV, which cleaved the DNA at a site located within the open reading frame (ORF) of crp. A ca. 900-bp SmaI fragment containing a streptomycin resistance cassette (str) was derived from p34S-Sm and was blunt end ligated into the EcoRV site to generate pGY365.
Construction of lacZYA reporter strains.
The yplA-lacZYA transcriptional fusion was described earlier (26). Construction of strain GY4641 (ysaE-lacZYA) will be described elsewhere (K. Venecia and G. M. Young, unpublished data). A lacZYA transcriptional fusion to flhB was constructed by cloning the promoter region into pFUSE followed by integration into the Y. enterocolitica chromosome as previously described (1). The ca. 580-bp DNA fragment containing the flhB promoter region (14) was designed to be flanked by XbaI and SmaI sites generated by PCR using oligonucleotide primers GYflhB1, 5′-GCT CTA GAG CAT AAC AAG GGT ATG GAG C-3′, and GYflhb2, TCC CCC GGG GGA TAT CTG GCC TTT CTC-3′. This allowed for the directional cloning of the fragment into corresponding sites in pFUSE.
Strain GY4455 containing a yscK-lacZYA fusion was isolated using a previously described procedure with some modifications (12). Y. enterocolitica JB580v was mutagenized with transposon TnMod-lacZYA-RKm′. Strains containing transposon insertions were isolated at 37°C as kanamycin-resistant colonies on MacConkey-lactose medium (Difco) that was rendered calcium limited by the addition of 20 mM sodium oxalate and 20 mM MgCl2. Calcium limitation induces the expression of the Ysc TTSS, which results in poor colony formation (12). The yscK::TnMod-lacZYA-RKm′ strain was identified as a strain that readily formed colonies and appeared red (lac+) on the selective medium. The location of the transposon insertion was determined by cloning and by determining the DNA sequence of the transposon/chromosomal junction region essentially as described above.
Mouse infection assays.
Evaluation of virulence by 50% lethal dose (LD50) analysis was completed as previously described (23). Virus-free, female, 5- to 7-week-old BALB/c mice were infected with selected strains of bacteria. Groups of six mice were infected perorally with successive 10-fold dilutions of a bacterial suspension (105 to 109 bacteria). The mice were monitored twice daily for 14 days. The day of death was recorded for each group of mice, and the LD50 was calculated according to the method of Reed and Muench (24).
The survival of bacteria in host tissues was examined by infecting groups of mice perorally with approximately 108 bacteria (23). At specified times mice were euthanatized and the number of bacteria present in selected tissues was determined. The Peyer's patches, mesenteric lymph nodes, spleens, and livers were aseptically removed from each mouse, weighed, and suspended in phosphate-buffered saline (23). Tissue samples were homogenized, and serial dilutions of the suspension were plated on LB medium containing nalidixic acid. The number of CFU per gram of infected tissue was calculated as previously described (23). All procedures were approved by the Animal Use and Care Administrative Advisory Committee of the University of California, Davis.
RESULTS
Isolation of mutants defective for Ysc, Ysa, and flagellar type III protein secretion.
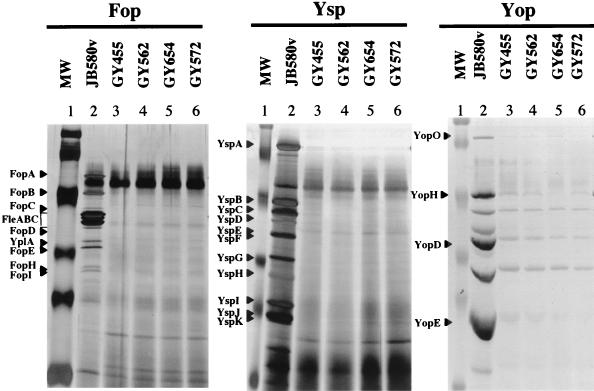
Y. enterocolitica was subjected to transposon mutagenesis with TnMod-RKm′ to identify mutants that exhibited a deficiency for the YplA export (Yex) phenotype on PLA medium. From approximately 40,000 transposon mutants, 77 were identified that had a Yex phenotype. YplA export is known to require the function of the flagellar TTSS under standard growth conditions (33, 34). As expected, 74 of the Yex mutants exhibited defects for motility and secretion of Fops (data not shown). To determine whether any Yex strains were also affected for protein secretion by the Ysc or Ysa TTSS, mutants were grown under conditions where Yops or Ysps are produced (Fig. 1). Examination of proteins secreted into the culture media revealed that mutants GY455, GY562, GY572, and GY654 produced significantly less Yops and did not appear to produce Ysps (Fig. 1). These mutants were further characterized in order to understand the nature of the defect that affected the three different TTSS. The other 70 mutants were saved for further characterization at a later date.
FIG. 1.
Analysis of secreted proteins by selected Y. enterocolitica strains under conditions that induce different TTSS. Extracellular proteins were concentrated from culture supernatants and separated by SDS-PAGE. Each strain was grown under conditions that induce the production of Fops, Ysps, or Yops as indicated above each panel. Lanes: 1, MW (molecular weight standards); 2, JB580v (wild type); 3, GY455 (Yex mutant); 4, GY562 (Yex mutant); 5, GY654 (Yex mutant); and 6, GY572 (Yex mutant). Cultures were grown under the following conditions: 26°C in L medium (1% tryptone and 0.5% yeast extract) devoid of added NaCl to induce the production of Fops, 26°C in L medium + 290 mM NaCl to induce the production of Ysps, and 37°C in L medium + 90 mM NaCl supplemented with 20 mM sodium oxalate and 20 mM MgCl2 to induce the production of Yops. Samples containing Fop and Ysp proteins were stained with silver. Yop proteins were stained with Coomassie brilliant blue. Each lane contains the equivalent of 1 ml of culture supernatant at an OD600 of 1.0. For each panel, labels indicating the locations of selected Fops and Ysps and Yops are assigned according to size on the left. Molecular mass standards are approximately 116, 80, 51.8, 34.7, 30, and 22 kDa.
Mutations in cya and crp affect type III protein secretion.
The site of the transposon insertion was determined for strains GY455, GY562, GY572, and GY654 in order to begin to understand the nature of the defect affecting Ysc, Ysa, and flagellar TTSS function. Characterization of the transposon/chromosome junction was facilitated by taking advantage of a pir-dependent origin of replication engineered within TnMod-RKm′ (11). Chromosomal DNA was isolated from each strain and treated with the endonuclease EcoRI. DNA fragments were then circularized by ligation with T4 DNA ligase, and transposon/chromosome fragments were recovered as autonomously replicating plasmids by tranformation of a pir+ strain of E. coli. The recovered plasmids were then analyzed to determine the DNA sequence of the transposon/chromosomal DNA junctions. Initial sequence analysis revealed that the transposon insertions were located in regions similar to two different loci present in E. coli. Two mutants had an insertion in a region that shared similarity to cya, and the other mutants had an insertion in a locus that shared similarity to crp. These genes, respectively, encode adenylate cyclase and CRP. Adenylate cyclase is necessary for the synthesis of the intracellular signaling molecule cAMP, which is recognized by CRP (3). The cAMP-CRP complex has both positive and negative regulatory functions that effect gene expression (3).
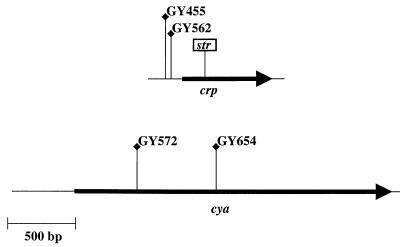
The cya and crp chromosomal regions of Y. enterocolitica were cloned, and the DNA sequence was determined (described in Materials and Methods). The proteins predicted to be encoded by Y. enterocolitica crp and cya exhibited a high degree of amino acid identity to corresponding proteins of other bacteria, including E. coli (97 and 84%, respectively) and Salmonella enterica (98 and 83%, respectively). This information allowed the precise location of the transposon insertions to be determined (Fig. 2). Strains GY572 and GY654 carried a transposon insertion located within the ORF of cya (Fig. 2). However, strains GY455 and GY562 had insertions within the predicted promoter region immediately upstream of crp (Fig. 2). To be sure that the phenotype of the crp mutants was due to a lack of expression, a new crp mutation was constructed that contained an insertion within the ORF (Fig. 2). The mutation was generated by cloning a streptomycin resistance cassette (str) into the EcoRV site of a plasmid-encoded copy of crp (described in Materials and Methods). The crp::str allele was then introduced into Y. enterocolitica by allelic exchange to generate strain GY4531. This crp mutant was examined for secretion of Yops, Ysps, and Fops and was found to exhibit phenotypes indistinguishable from those of the transposon mutants (further described below). The strain did not secrete TTSS-dependent proteins under conditions that normally induce the Ysc, Ysa, or flagellar TTSS (data not shown). In addition, the crp::str mutant was not motile (data not shown).
FIG. 2.
Schematic diagram of the crp and cya regions of the Y. enterocolitica chromosome that were cloned. The location and orientation of the ORF are indicated for each gene by the black arrow. The location of the transposon insertion harbored by the Yex mutants is indicated by the flag and labeled with the corresponding name of the strain. The location of the streptomycin resistance cassette (str) recombined into the chromosome of several different strains examined is also shown.
Complementation of cya and crp mutations restores type III protein secretion.
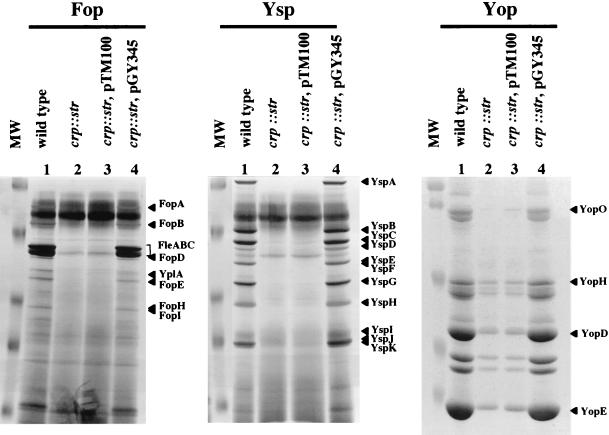
The available plasmid-encoded clones of cya and crp provided the opportunity to introduce a functional allele into the transposon mutants. Each mutant was transformed with either the cloning vector or the appropriate plasmid clone. The strains were examined for the ability to produce Yops, Ysps, and Fops (Fig. 3). Complementation of the cya and crp mutations with a plasmid-encoded copy of the respective gene restored the production of secreted proteins when cultures were grown under conditions that induce the Ysc, Ysa, or flagellar TTSS (Fig. 3). The cloning vector had no effect on the phenotype of the mutants. Likewise, complementation restored motility to each mutant (data not shown). Similar results were observed when the crp::str mutant was examined, indicating that the observed phenotypes are not allele specific (Fig. 4). These results indicate that the defects for Yop, Ysp, and Fop secretion by these Yex mutants were due to loss of cya and crp function.
FIG.3.
Analysis of secreted proteins by selected Y. enterocolitica strains. Secreted proteins were isolated from cultures of strains grown under different conditions to induce the production of Fops, Ysps, or Yops and were analyzed as indicated for Fig. 1. Lanes: 1, molecular weight standards; 2, JB580v (wild type); 3, GY572 (cya); 4, GY572/pTM100 (vector control); 5, GY572/pGY355 (cya+); 6, GY455 (crp); 7, GY455/pTM100 (vector control); and 8, GY455/pGY345 (crp+). Each lane contains the equivalent of 1 ml of culture supernatant at an OD600 of 1.0. For each panel, labels indicating the locations of selected Fops, Ysps, and Yops are assigned according to size on the right. Molecular mass standards are approximately 116, 80, 51.8, 34.7, 30, and 22 kDa. Proteins were visualized as indicated for Fig. 1.
FIG.4.
Analysis of secreted proteins by the crp::str mutant of Y. enterocolitica. Secreted proteins were isolated from cultures of indicated strains grown under different conditions to induce the production of Fops, Ysps, or Yops and analyzed as indicated for Fig. 1. Lanes: 1, JB580v (wild type); 2, GY4531 (crp::str); 3, GY4531/pTM100 (vector control); and 4, GY4531/pGY345 (crp+). Each lane contains the equivalent of 1 ml of culture supernatant at an OD600 of 1.0. For each panel, labels indicating the location of selected Fops, Ysps, and Yops are assigned according to size on the right. Molecular mass standards are approximately 116, 80, 51.8, 34.7, 30, and 22 kDa. Proteins were visualized as indicated for Fig. 1. MW, molecular weight.
The requirement of crp for expression of type III secretion genes.
The defect for secretion of Yop, Ysp, and Fop secretion suggested that crp may be required for optimal expression of genes encoding components of the Ysc, Ysa, and flagellar TTSS. To begin to examine this possibility, strains were constructed that contained lacZYA transcriptional fusions to yscK, ysaE, and flhB, which encode components of the Ysc, Ysa, and flagellar secretion apparatus, respectively (Table 1; described in Materials and Methods). The crp::str allele was introduced into each of these reporter strains. Strains carrying a yplA-lacZYA transcriptional fusion were also examined. Reporter stains that had a functional or mutated copy of crp were grown under laboratory conditions known to induce the corresponding TTSS, and the levels of β-galactosidase activity were determined (Table 2). The results showed that inactivation of crp led to reduced expression of yscK, ysaE, flhB, and yplA (Table 2). Complementation analysis revealed that loss of expression due to the crp mutation could be alleviated by introduction of a functional copy of crp (Table 2). This suggests that reduced production of Yop, Ysp, and Fops by crp mutants is probably due to reduced expression of genes required for the function of the corresponding protein secretion systems.
TABLE 2.
The effect of crp mutations on the expression of selected genes involved in type III protein secretiona
| Relevant genotypeb | Plasmid | β-Galactosidase activityc |
|---|---|---|
| flhB-lacZYA | 260 ± 14 | |
| flhB-lacZYA, crp | 3 ± 1 | |
| flhB-lacZYA, crp | pTM100 | 3 ± 1 |
| flhB-lacZYA, crp | pGY345 (crp+) | 197 ± 9 |
| ysaE-lacZYA | 270 ± 1 | |
| ysaE-lacZYA, crp | 30 ± 1 | |
| ysaE-lacZYA, crp | pTM100 | 23 ± 1 |
| ysaE-lacZYA, crp | pGY345 (crp+) | 393 ± 4 |
| yscK-lacZYA | 3,887 ± 172 | |
| yscK-lacZYA, crp | 1,475 ± 51 | |
| yscK-lacZYA, crp | pTM100 | 1,416 ± 60 |
| yscK-lacZYA, crp | pGY345 (crp+) | 3,710 ± 287 |
| yplA-lacZYA | 555 ± 52 | |
| yplA-lacZYA, crp | 38 ± 1 | |
| yplA-lacZYA, crp | pTM100 | 25 ± 5 |
| yplA-lacZYA, crp | pGY345 (crp+) | 864 ± 41 |
Overnight cultures of each strain were subcultured to an OD600 of 0.1 in fresh media and were grown under conditions known to induce the expression of the specified gene. Inducing conditions used were L medium at 26°C (flhB and yplA), L medium + 300 mM NaCl at 26°C (ysaE), and L medium + 20 mM sodium oxalate + 20 mM MgCl2 at 37°C (yscK).
Strains are described in Table 1.
Units of β-galactosidase activity are expressed in Miller units. Assays were performed in duplicate on at least three different cultures. Samples were collected and assayed at multiple time points following subculture. Results for samples collected at 2 h (ysaE and yscK) or 6 h (flhB and yplA) are reported; they represent the time point where expression is greatest for each gene examined. However, similar results were obtained at other time points throughout the course of the experiment.
An essential role for cya and crp in virulence.
The secretion defects of the cya and crp mutants suggested that these strains might be affected for virulence. This possibility was examined using the murine model of infection (5). BALB/c mice were perorally infected with Y. enterocolitica JB580v, GY455, or GY572 to determine the ability of these strains to cause a lethal infection (Table 3). The results of this analysis indicated that the mutants were attenuated for virulence. Mice were generally resistant to developing a lethal infection when inoculated with either the crp or the cya mutant, even at a dose exceeding 109 CFU (Table 3). Furthermore, the calculated LD50 was 5 × 107 CFU for JB580v but could only be estimated to be > 5 × 109 CFU for each mutant, since nearly all of the mice survived the challenge (Table 3).
TABLE 3.
Mortality of BALB/c mice 14 days after oral inoculation with Y. enterocoliticaa
| Strain | Relevant genotype | Inoculating dose (CFU) | No. of survivors/ total no. of mice |
|---|---|---|---|
| JB580v | Wild type | 1 × 109 | 0/6 |
| 1 × 108 | 3/6 | ||
| 1 × 107 | 4/6 | ||
| 1 × 106 | 6/6 | ||
| 1 × 105 | 6/6 | ||
| GY572 | cya | 7 × 109 | 6/6 |
| 7 × 108 | 6/6 | ||
| 7 × 107 | 5/6 | ||
| 7 × 106 | 5/6 | ||
| 7 × 105 | 6/6 | ||
| GY455 | crp | 7 × 109 | 4/6 |
| 7 × 108 | 6/6 | ||
| 7 × 107 | 6/6 | ||
| 7 × 106 | 6/6 | ||
| 7 × 105 | 6/6 |
Mice were inoculated perorally with indicated strains. Morbidity and mortality were observed for 14 days.
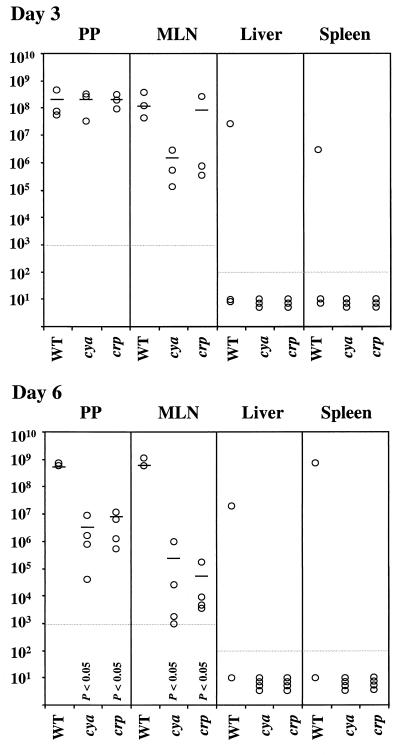
To complement LD50 analysis, the number of bacteria present in selected host tissues was determined at specific time points following oral inoculation of mice (Fig. 5). For these experiments, mice were perorally infected with strain JB580v, GY455, or GY572. The number of bacteria that were present in Peyer's patches, mesenteric lymph nodes, livers, and spleens of mice after 3 and 6 days of infection was determined. At day 3 of infection, the wild-type and mutant strains of Y. enterocolitica were present in Peyer's patches and mesenteric lymph nodes at similar levels (Fig. 5). However, there were significant differences after 6 days of infection (Fig. 5). Only two of the three mice infected with JB580v survived, and tissues from the surviving mice generally had large numbers of bacteria (Fig. 5). In contrast, no bacteria were detected in livers or spleens of mice infected with the cya and crp mutants (Fig. 5). These results suggest that cya and crp mutants are able to initiate an infection of mice but are restricted to Peyer's patches and mesenteric lymph nodes.
FIG. 5.
The number of bacteria recovered from tissues of mice perorally infected with Y. enterocolitica. A group of six mice were infected with 108 CFU of JB580v (wild type [WT]). Groups of seven mice were infected with 108 CFU of GY455 (crp) and GY572 (cya). On day 3, the number of bacteria recovered per gram of tissue for three mice from each group was determined. On day 6, the number of bacteria recovered per gram of tissue for the remaining mice from each group was determined. Note that one mouse from the group infected with wild-type Y. enterocolitica died at 51/2 days. The data from this mouse are not plotted. No bacteria were recovered from tissues where the value is plotted below the limits of detection. The limit of detection is indicated as a gray horizontal line on each scatter plot. The mean number of CFU per gram of tissue was calculated for data from Peyer's patches (PP) and mesenteric lymph nodes (MLN) and is indicated by a horizontal bar. Data from samples obtained from mice infected with mutant bacteria were compared to those from the mice infected with wild-type bacteria by using Student's t test. P is shown when the mean number of bacteria from mice infected with mutant bacteria is significantly different from the wild-type number.
Since cya and crp mutant strains initiate an infection and survive in Peyer's patches and mesenteric lymph nodes, these strains may lead to an immune response that is protective against fully virulent Y. enterocolitica. As a preliminary test to evaluate the effectiveness of crp and cya mutants at stimulating protective immunity, groups of six mice were given a single oral dose of strain GY455 or GY572 (Table 4). The animals were monitored for 14 days and did not appear to develop disease symptoms. Each mouse was then challenged with a single oral dose of the virulent strain JB580v (1.4 × 109 CFU). Untreated mice develop a lethal infection with an average time to death of approximately 7 days. The immunized mice were followed for an additional 14 days after the challenge with virulent Y. enterocolitica. During this period of observation the animals did not exhibit any overt signs of infection, suggesting that the cya and crp mutants of Y. enterocolitica were effective at stimulating a protective immunity that prevented mice from developing a lethal infection by the virulent strain. The nature of this protective immune response, the longevity of immune protection, and the kinetics of bacterial clearance will require additional experiments and will be the subject of a future study.
TABLE 4.
Effectiveness of single oral immunization with Y. enterocolitica mutants in protecting BALB/c mice against challenge with a virulent straina
| Strain | Relevant genotype | Immunizing dose (CFU) | Challenging dose (CFU) | No. of survivors/ total no. of mice |
|---|---|---|---|---|
| GY572 | cya | 6 × 108 | 1.4 × 109 | 6/6 |
| GY455 | crp | 6 × 108 | 1.4 × 109 | 6/6 |
Mice were inoculated perorally with a single dose of the indicated strain. After 14 days they were challenged with wild-type Y. enterocolitica JB580v. Morbidity and mortality were observed for an additional 14 days postchallenge.
DISCUSSION
Many Y. enterocolitica virulence factors have been identified that are expressed under a variety of different conditions in the laboratory. The mechanism underlying the regulation of different virulence factors may provide a way to conserve resources and to ensure that virulence factors are expressed in an appropriate fashion to support survival during infection. Different virulence factors may contribute to particular aspects of infection, and the complexity of in vitro patterns of virulence factor expression may reflect the different conditions encountered by the pathogen during the course of infection. Achieving the fine-tuned response of the bacterium to the host environment is likely to involve both global regulatory systems and systems that specifically modulate a subset of activities.
In this study, the evidence presented suggests that adenylate cyclase and CRP are required for normal expression of virulence genes. Adenylate cyclase catalyzes the formation of cAMP, which is a common global regulatory molecule in biological systems (3). Intracellular cAMP forms a complex with its receptor CRP that induces sequence-specific DNA binding (3). cAMP-CRP can function both as a positive and negative regulator of gene expression in enteric bacteria. This is the first examination of the role of the cAMP-CRP regulatory system in Y. enterocolitica virulence. The cya and crp genes were identified because they are necessary for production of the virulence factor YplA, which is secreted by the flagellar TTSS. Subsequent analysis revealed that these genes are also required for the production of proteins secreted by the Ysc and Ysa TTSS.
Initial phenotypic analysis suggested that the cAMP-CRP regulatory system was required for the expression of genes required for TTSS function. An examination of gene expression using transcriptional fusions to lacZ revealed that at least some genes encoding components of the Ysc, Ysa, and flagellar TTSS require a functional copy of crp for expression. This analysis does not reveal the actual targets of the cAMP-CRP complex. It is possible that cAMP-CRP may directly or indirectly affect the expression of genes encoding components of Ysc, Ysa, and flagellar TTSS. The analysis also suggests that the cAMP-CRP regulatory system could influence the expression of other virulence factors. However, the effects of this system do not extend to all virulence factors. We have tested the possibility that cya and crp mutants might be affected for the production of urease or invasin, but the results showed that the mutants were indistinguishable from the wild-type strain (data not shown). Nonetheless, this study has revealed that global regulatory systems can play a role in controlling virulence factors that may act at different stages of an infection.
An evaluation of cya and crp mutants of Y. enterocolitica for virulence using a mouse model of infection also supported a role for cAMP-CRP in controlling the expression of essential virulence factors. Neither cya nor crp mutants caused significant morbidity when mice were infected with a dose at least 100 times the LD50 for virulent Y. enterocolitica. Attenuation could be due to altered regulation of virulence factors and changes in the metobolic capacity of cya and crp mutants. Studies of the role of cAMP-CRP in other bacteria have shown that this regulatory system plays an important role in controlling metabolic pathways (3). Although we cannot rule out the possibility that cya and crp mutants multiply more slowly in the host, both mutants retained the ability to invade the host and gain access to Peyer's patches and mesenteric lymph nodes. Survival of the mutants was not significantly different from that of virulent Y. enterocolitica within these lymphoid tissues for 3 days. Mutant bacteria were also recovered from Peyer's patches and mesenteric lymph nodes after 6 days of infection. This suggests that a general defect in Y. enterocolitica metobolic pathways is probably not a primary cause for attenuation of cya and crp mutants. The observed attenuation might indicate that the in vitro defects in the function of the three different TTSS of Y. enterocolitica accurately reflect altered functioning of these protein secretion systems during an infection.
Virulence gene regulation in a number of different enteric pathogens, including S. enterica (9) and Vibrio cholerae (28), has been shown to be affected by the cAMP-CRP system. For some S. enterica serotypes, cya and crp mutants have been useful as live oral vaccine strains (9). A preliminary examination of the potential of cya and crp mutants of Y. enterocolitica for use as live oral vaccine strains in mice revealed that a single oral dose was sufficient to immunize animals to subsequent challenge with virulent bacteria. We note that this was a preliminary short-course analysis. Additional studies are required to characterize the mechanism of this protective immune response and to determine if immunization can lead to long-term protection. In addition, it will be interesting to determine if cya and crp mutants of Y. enterocolitica can be used as live attenuated carrier vaccine vectors to promote immunity to other pathogenic species of Yersinia or other infectious agents. At present there is no well-defined oral vaccine for Y. enterocolitica, and the similarities between this bacterium and the plague bacterium, Y. pestis, suggest that development of a cross-protective vaccine is possible.
Acknowledgments
We thank Dave Hendrixson and Briana Young for critical review of the manuscript. We also thank Krista Venecia and Andrew Darwin for providing strains.
This work was supported by University of California startup funds and an Academic Senate Faculty Research Award.
Editor: V. J. DiRita
REFERENCES
- 1.Baumler, A. J., R. M. Tsolis, A. W. van der Velden, I. Stojiljkovic, S. Anic, and F. Heffron. 1996. Identification of a new iron regulated locus of Salmonella typhi. Gene 183:207-213. [DOI] [PubMed] [Google Scholar]
- 2.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 3.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56:100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottone, E. J. 1999. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1:323-333. [DOI] [PubMed] [Google Scholar]
- 5.Carter, P. B., and F. M. Collins. 1974. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect. Immun. 9:851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, L. W., and O. Schneewind. 2000. Type III machines of Gram-negative bacteria: delivering the goods. Trends Microbiol. 8:214-220. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis, G., Y. Laroche, G. Balligand, M.-P. Sory, and G. Wauters. 1987. Y. enterocolitica, a primary model for bacterial invasiveness. Rev. Infect. Dis. 9:64-87. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriate, C. Neyt, M.-P. Sory, and I. Stainer. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtiss, R., III, and S. M. Kelly. 1987. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect. Immun. 55:3035-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darwin, A. J., and V. L. Miller. 1999. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol. Microbiol. 32:51-62. [DOI] [PubMed] [Google Scholar]
- 11.Dennis, J. J., and G. J. Zylstra. 1998. Plaposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goguen, J. D., J. Yother, and S. C. Straley. 1984. Genetic analysis of the low calcium response in Yersinia pestis Mu d1(Ap lac) insertion mutants. J. Bacteriol. 160:842-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:1436-1446. [DOI] [PubMed] [Google Scholar]
- 14.Heusipp, G., G. M. Young, and V. L. Miller. 2001. HreP, an in vivo-expressed protease of Yersinia enterocolitica, is a new member of the family of subtilisin/kexin-like proteases. J. Bacteriol. 183:3556-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iriarte, M., and G. R. Cornelis. 1996. Molecular determinants of Yersinia pathogenesis. Microbiologia 12:267-280. [PubMed] [Google Scholar]
- 16.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O:8 and construction of a transformable R−M+ mutant. Gene 136:271-275. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd, S. A., A. Forsberg, H. Wolf-Watz, and M. S. Francis. 2001. Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol. 9:367-371. [DOI] [PubMed] [Google Scholar]
- 18.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Michiels, T., J.-C. Vanooteghem, C. Lambert de Rouvroit, B. China, A. Gustin, P. Boudry, and G. Cornelis. 1991. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 173:4994-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michiels, T., P. Wattiau, R. Brasseur, J.-M. Ruysschaert, and G. Cornelis. 1990. Secretion of Yop proteins by yersiniae. Infect. Immun. 58:2840-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 22.Pepe, J. C., J. L. Badger, and V. L. Miller. 1994. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol. Microbiol. 11:123-135. [DOI] [PubMed] [Google Scholar]
- 23.Pepe, J. C., and V. L. Miller. 1993. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc. Natl. Acad. Sci. USA 90:6473-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 25.Schmiel, D. H., E. Wagar, L. Karamanou, D. Weeks, and V. L. Miller. 1998. Phospholipase A of Yersinia enterocolitica contributes to pathogenesis in a mouse model. Infect. Immun. 66:3941-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmiel, D. S., G. M. Young, and V. L. Miller. 2000. The Yersinia enterocolitica phospholipase gene yplA is part of the flagellar regulon. J. Bacteriol. 182:2314-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 28.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 94:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skurnik, M., and P. Toivanen. 1993. Yersinia enterocolitica lipopolysaccharide: genetics and virulence. Trends Microbiol. 1:148-152. [DOI] [PubMed] [Google Scholar]
- 30.Straley, S. C. 1991. The low-Ca2+ response virulence regulon of human-pathogenic Yersiniae. Microb. Pathog. 10:87-91. [DOI] [PubMed] [Google Scholar]
- 31.Straley, S. C., and R. D. Perry. 1995. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 3:310-317. [DOI] [PubMed] [Google Scholar]
- 32.Trotot, P., O. Sismeiro, C. Vivares, P. Glaser, A. Bresson-Roy, and A. Danchin. 1996. Comparative analysis of the cya locus in enterobacteria and related gram-negative facultative anaerobes. Biochimie 78:277-287. [DOI] [PubMed] [Google Scholar]
- 33.Young, B. M., and G. M. Young. 2002. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 184:1324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young, G. M., M. Smith, S. A. Minnich, and V. L. Miller. 1998. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 181:2823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]