Abstract
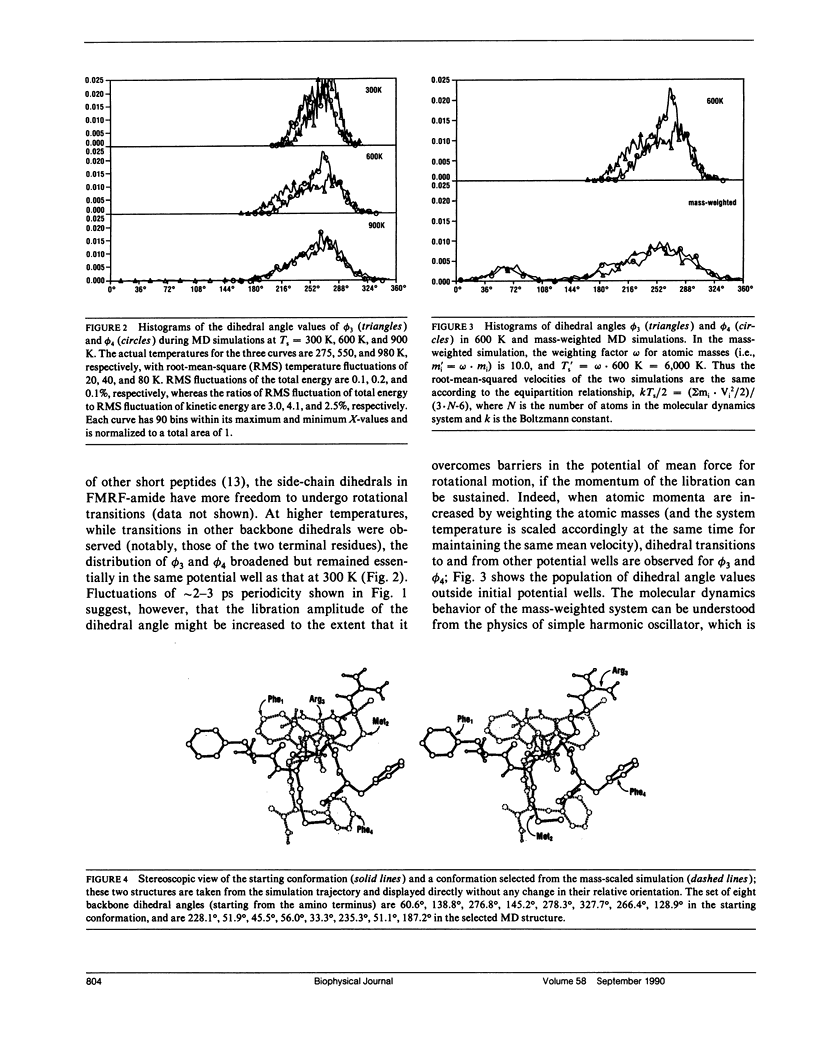
A molecular dynamics-based simulation method in which atomic masses are weighted is described. Results from this method showed that the capability for conformation search in molecular dynamics simulation of a short peptide (FMRF-amide) is significantly increased by mass weighting.
Full text
PDF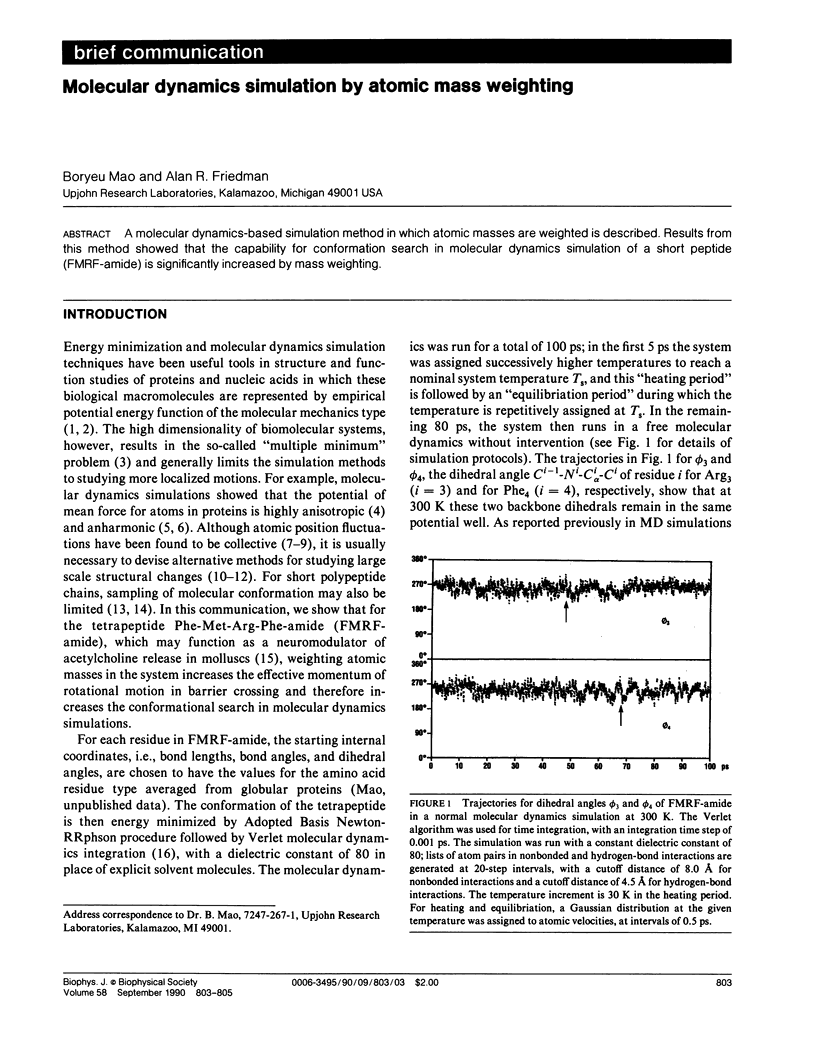

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks B., Karplus M. Harmonic dynamics of proteins: normal modes and fluctuations in bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6571–6575. doi: 10.1073/pnas.80.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go N., Noguti T., Nishikawa T. Dynamics of a small globular protein in terms of low-frequency vibrational modes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3696–3700. doi: 10.1073/pnas.80.12.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler A. T., Osguthorpe D. J., Dauber-Osguthorpe P., Hempel J. C. Dynamics and conformational energetics of a peptide hormone: vasopressin. Science. 1985 Mar 15;227(4692):1309–1315. doi: 10.1126/science.3975616. [DOI] [PubMed] [Google Scholar]
- Harvey S. C., McCammon J. A. Intramolecular flexibility in phenylalanine transfer RNA. Nature. 1981 Nov 19;294(5838):286–287. doi: 10.1038/294286a0. [DOI] [PubMed] [Google Scholar]
- Ichiye T., Karplus M. Anisotropy and anharmonicity of atomic fluctuations in proteins: analysis of a molecular dynamics simulation. Proteins. 1987;2(3):236–259. doi: 10.1002/prot.340020308. [DOI] [PubMed] [Google Scholar]
- Kitson D. H., Hagler A. T. Theoretical studies of the structure and molecular dynamics of a peptide crystal. Biochemistry. 1988 Jul 12;27(14):5246–5257. doi: 10.1021/bi00414a045. [DOI] [PubMed] [Google Scholar]
- Levy R. M., Sheridan R. P., Keepers J. W., Dubey G. S., Swaminathan S., Karplus M. Molecular dynamics of myoglobin at 298 degrees K. Results from a 300-ps computer simulation. Biophys J. 1985 Sep;48(3):509–518. doi: 10.1016/S0006-3495(85)83806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man-Son-Hing H., Zoran M. J., Lukowiak K., Haydon P. G. A neuromodulator of synaptic transmission acts on the secretory apparatus as well as on ion channels. Nature. 1989 Sep 21;341(6239):237–239. doi: 10.1038/341237a0. [DOI] [PubMed] [Google Scholar]
- Mao B., Pear M. R., McCammon J. A., Northrup S. H. Molecular dynamics of ferrocytochrome c: anharmonicity of atomic displacements. Biopolymers. 1982 Oct;21(10):1979–1989. doi: 10.1002/bip.360211005. [DOI] [PubMed] [Google Scholar]
- Mao B., Pear M. R., McCammon J. A., Quiocho F. A. Hinge-bending in L-arabinose-binding protein. The "Venus's-flytrap" model. J Biol Chem. 1982 Feb 10;257(3):1131–1133. [PubMed] [Google Scholar]
- Northrup S. H., Pear M. R., Morgan J. D., McCammon J. A., Karplus M. Molecular dynamics of ferrocytochrome c. Magnitude and anisotropy of atomic displacements. J Mol Biol. 1981 Dec 25;153(4):1087–1109. doi: 10.1016/0022-2836(81)90469-1. [DOI] [PubMed] [Google Scholar]
- Thaisrivongs S., Mao B., Pals D. T., Turner S. R., Kroll L. T. Renin inhibitory peptides. A beta-aspartyl residue as a replacement for the histidyl residue at the P-2 site. J Med Chem. 1990 May;33(5):1337–1343. doi: 10.1021/jm00167a009. [DOI] [PubMed] [Google Scholar]


