Abstract
We previously demonstrated that interleukin-1 (IL-1) and tumor necrosis factor (TNF) activities only partially account for calvarial bone resorption induced by local application of lipopolysaccharide (LPS) in mice. The present study was undertaken to determine the role and relative contribution of IL-11 and prostaglandin(s) (PG[s]) in LPS-induced bone resorption in vivo. A one-time dose of LPS was injected into the subcutaneous tissue overlying calvaria of mice lacking IL-1 receptor type I (IL-1RI−/−), mice lacking TNF receptor p55 and IL-1RI (TNFRp55−/−-IL-1RI−/−), and wild-type mice. Mice were then treated with injections of anti-IL-11 monoclonal antibody (MAb), indomethacin, or phosphate-buffered saline (PBS) and sacrificed 5 days later. Histological sections stained for tartrate-resistant acid phosphatase (TRAP) were quantified by histomorphometric analysis. At low doses of LPS (100 μg/mouse), the percentages of bone surface covered by osteoclasts were found to be similar in three strains of mice. The increase was reduced by 37% with anti-IL-11 MAb and by 46% with indomethacin. At higher doses of LPS (500 μg/mouse), we found an eightfold increase in these percentages in wild-type mice and a fivefold increase in these percentages in IL-1RI−/− and TNFRp55−/−−IL-1RI−/− mice after normalizing with the value from the saline-PBS control group in the same strain of mice. The increase was reduced by 55 and 69% in wild-type mice and by 50 and 57% in IL-1RI−/− and TNFRp55−/−−IL-1RI−/− mice treated with anti-IL-11 MAb or indomethacin, respectively. Our findings suggest that in vivo, at low doses of LPS (100 μg/mouse), LPS-induced bone resorption is mediated by IL-11 and PGs, while at high doses of LPS (500 μg/mouse), it is mediated by IL-11, PGs, IL-1, and TNF signaling. IL-11 and PGs mediate LPS-induced bone resorption by enhancing osteoclastogenesis independently of the IL-1 or TNF signaling.
Bone resorption is a feature of chronic inflammatory diseases such as rheumatoid arthritis, osteomyelitis, bacterial arthritis, and periodontitis (16). Periodontitis is a common inflammatory disorder that often leads to irreversible alveolar bone resorption and tooth loss among adults and is characterized as a peripheral infection involving species of gram-negative organisms (37). The key issue to be addressed in these diseases is how bacteria cause pathological bone loss. Lipopolysaccharide (LPS), a component of the outer membranes of all gram-negative bacteria (28), was the first bacterial component shown to be capable of inducing bone resorption in vitro (16). Although LPS has been identified as a major bacterial bone-resorbing factor, the cellular mechanism by which LPS stimulates bone resorption has not been fully elucidated. Understanding the means by which LPS enhances bone resorption would provide an effective therapeutic strategy to prevent and treat bacterially induced bone resorption.
It is known that LPS triggers the inflammatory process both locally and systemically and stimulates cytokine secretion (16, 38). LPS may stimulate host cells, including gingival fibroblasts, fibroblastic cells in the periodontal ligament, recruited leukocytes (monocytes and macrophages), or osteoblasts producing cytokines and local mediators (16, 27, 32, 35). The major effects of LPS are produced by mediators (e.g., LPS-binding proteins, cytokines, prostaglandins, prostacyclins, and NO, etc.). But the signal pathway by which LPS stimulates bone resorption is unclear.
Our previous study (5) demonstrated the existence of two signal pathways in LPS-induced bone resorption in vivo: one at high LPS doses and one at low LPS doses. At higher concentrations of LPS, osteoclastogenesis and bone resorption are substantially mediated by interleukin-1 (IL-1) and tumor necrosis factor (TNF) receptor signaling, but this signaling does not completely account for bone resorption at lower LPS concentrations. At lower LPS concentrations, additional mediators, working independently or in concert with IL-1 and TNF, appear to contribute to LPS-induced bone resorption in vivo. The present work is a follow-up of our previous study advocating for the role of IL-11 and prostaglandin(s) (PG[s]) with IL-1 and TNF in LPS-induced bone resorption in an in vivo mouse model.
There are two forms of biologically active IL-1 (α and β forms) and two homologous receptors for IL-1 (IL-1 type I receptor [IL-1RI] and type II receptor [IL-1RII]) that can bind either form of IL-1, although with different affinities (2). The type I receptor is capable of mediating a biological signal, while the type II receptor is thought to function as a decoy receptor (34). Two types of high-affinity receptors, p55 and p75, have been identified for TNF molecules. Most but not all TNF activity has been shown to be mediated by the TNF receptor p55 (TNFRp55) (25, 30, 31). Recent evidence obtained from mice lacking p75 suggests that p75 may act to suppress TNF-mediated inflammatory responses (31).
IL-11, a 178-amino-acid nonglycosylated peptide cytokine critical for osteoclast development (9), was initially isolated from a bone marrow-derived stromal cell line (24). IL-11 belongs to a family of cytokines including IL-6, leukemia inhibitory factor, oncostatin M, ciliary neurotrophic factor, and cardiotrophin 1, all of which signal through gp130 (12). IL-11 is not produced by monocytes or lymphocytes. Its expression is restricted to certain cells of the mesenchymal lineage, such as bone marrow stromal cells, osteoblasts (29), articular chondrocytes, synoviocytes, lung fibroblasts (15), and osteosarcoma cells (8). IL-11 receptor transcripts have been demonstrated on mature osteoclasts and osteoblasts in bone marrow cultures (29) and on osteoblastic and chondroblastic progenitor cells during mouse embryogenesis (17). Current research demonstrated that IL-11 stimulates multinucleated osteoclast-like cell formation and bone resorption in a dose-dependent manner in cell and organ culture systems (9, 11, 29).
PGs are ubiquitous local hormones that have long been known to exert important effects on the skeleton (26) and in immuno-inflammatory responses. Extensive clinical research over many years has also demonstrated a significant role of PGs in the pathogenesis of periodontal disease (13, 14, 18, 19). Elevated PGE2 levels within the gingival crevicular fluid have been suggested as a reliable biochemical marker for periodontal disease activity and severity (18-20). PGs are produced as the metabolites from arachidonic acid by two key enzymes, cyclooxygenase 1 and cyclooxygenase 2. Cyclooxygenase 1 is produced constitutively in many tissues and appears to represent an essential component of tissue homeostasis. In contrast, cyclooxygenase 2 is generally undetectable under physiological conditions and appears to be responsible for PGs in inflammation. Indomethacin is a relatively nonselective inhibitor of both types of cyclooxygenase (40) and has been widely used in in vitro and in vivo studies.
Interestingly, the systematic role and relative contribution of IL-11 and PGs (compared to TNF and IL-1) in the regulation of LPS-induced bone resorption in vivo remains unknown. Therefore, by using anti-IL-11 monoclonal antibody (MAb) and indomethacin, we investigated in the present study the relative role of IL-11 and PGs in the overall process of bone resorption at high and low doses of LPS in three strains of mice. Our data indicated that IL-11 and PGs are significant mediators of LPS-induced osteoclastogenesis and bone resorption in vivo and that they may act independently of IL-1 and TNF receptor signaling.
MATERIALS AND METHODS
Reagents and mice.
Escherichia coli serotype O55:B5 LPS (catalog no. L2880; Sigma, St. Louis, Mo.) was dissolved in phosphate-buffered saline (PBS) (5 mg/ml) by sonication for 2 min, aliquoted, and stored at −80°C until use. Before each injection, the stock solution was sonicated for 2 min again. Anti-IL-11 MAb 11 h3/19.6.1. (murine immunoglobulin G1), a gift of Genetics Institute, Inc. (Cambridge, Mass.), was freshly prepared at the concentration of 9.3 mg/ml in PBS before each injection according to Genetic Institute recommendations. Indomethacin (1-[p-chlorobenzoyl]-5-methoxy-2-methylindole-3-acetic acid Sigma) was suspended at 1 mg/ml immediately prior injection (4).
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine. Three strains of mice were used in this study: transgenic mice with a targeted deletion of IL-1 receptor type I (IL-1RI−/−) and TNF receptor p55 and IL-1RI double knockouts (TNFRp55−/−-IL-1RI−/−) were generously provided by J. Peschon (Immunex Corp., Seattle, Wash). Genetically matched wild-type C57BL/6 ×129 hybrids were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were between 8 and 12 weeks of age during the study. Regular mouse chow and water were provided ad libitum. The genotypes of mutant mice were routinely confirmed by PCR of extracted DNA. Each strain of mice was randomly assigned into a group with a low dose of LPS (100 μg/mouse) or a high dose of LPS (500 μg/mouse). Each group included four different arms: (i) LPS challenge followed by anti-IL-11 MAb (LPS-anti-IL-11), (ii) LPS challenge followed by indomethacin, (iii) LPS challenge followed by PBS (LPS-PBS), and (iv) saline challenge followed by PBS (saline-PBS), which served as a control. There were seven mice in each group.
Calvarial bone injection.
The injections were administered with a 30.5-gauge needle at a point on the midline of the skull located between the ears and eyes. LPS, anti-IL-11 MAb, indomethacin, or PBS was delivered in the space between the subcutaneous tissue and the periosteum of the skull.
Prior to the first injection, all animals were anesthetized intraperitoneally with a ketamine-xylazine solution (a combination of 1 ml of ketamine [Ketaset], 1 ml of xylazine [Rompum; Fisher, Columbus, Ohio], and 6 ml of sterile PBS [Gibco BRL, Grand Island, N.Y.]). Approximately 5 μl of anesthetic per gram of body weight was administered. The heads of the anesthetized mice were shaved. One dose of LPS (100 or 500 μg/mouse) or saline, each in a 100-μl volume, was injected subcutaneously and then immediately followed by a same-site injection (50 μl/mouse) of anti-IL-11 MAb (465 μg/mouse), indomethacin (50 μg/mouse), or PBS. For subsequent treatments, all mice were anesthetized via inhaled isoflurane prior to their injections. Thereafter, anti-IL-11 MAb (465 μg/mouse) or PBS, each in a volume of 50 μl/mouse, was administered subcutaneously every 4 h for a total of four injections (total dose of 1.86 mg of anti-IL-11/mouse). Indomethacin (50 μg/mouse) or PBS was injected in a 50-μl volume every 8 h, for a total of nine injections (total dose of 450 μg of indomethacin/mouse). Mice were sacrificed in a CO2 chamber 5 days after the first injection.
Specimen preparation.
The entire calvarial bone together with the overlying skin tissue was dissected and fixed in freshly prepared paraformaldehyde (4% in PBS, pH 7.2) for 6 h at 4°C. The specimen was then washed with 5, 10, and 15% glycerol in PBS, each for 15 min, and decalcified in Immunocal solution (Decal Corporation, Congers, N.Y.) for 3 days at 4°C with gentle stirring. After two washes with Immunocal Neutralizer Solution (Decal Corporation) for 20 min at 4°C, the anterior half of the frontal bone and the posterior half of the occipital bone were trimmed off. The remaining calvarial bone, with its overlying skin tissue, was immersed in 30% sucrose in PBS overnight and then transferred into −80°C prechilled 2-methyl-butane (Sigma, St. Louis, Mo.) and stored at −80°C until embedding.
Histochemical staining.
The calvarial bone with skin tissue was cut in half through the sagittal suture. The two halves were embedded side by side with HISTO PREP compound (Fisher Scientific, Hanover Park, Ill.). Serial sections (5 μm) were made by cryostat sectioning. Fifty slides were obtained for each specimen, and every 10th slide was kept for tartrate-resistant acid phosphatase (TRAP) staining. The TRAP staining solution was prepared as follows: 9.6 mg of naphthol AS-BI phosphate substrate (Sigma) was dissolved in 0.6 ml of N,N-dimethylformamide (Sigma) with 60 ml of 0.2 M sodium acetate buffer (pH 5.0; Sigma), which contained 84 mg of fast red-violet LB diazonium salt (Sigma), 58.2 mg of tartaric acid (Sigma), and 240 μl of 10% MgCl2. The mixture was filtered through a 0.22-μm-pore-size filter. Slides were incubated for 8 min in the staining solution at 37°C in the dark. The slides were then washed with water for 30 min, which was followed by counterstaining with hematoxylin for 5 to 6 min.
Bone histomorphometry.
For each animal, four slides, each containing two tissue sections with the largest number of osteoclasts (criteria described below) were analyzed by computer-assisted image analysis (Image Pro plus image software 4.0; Media Cybernetics, Silver Spring, Md.). The bone surfaces in the three sutures (the coronal suture, the lambdoid suture and the suture between the interparietal and occipital bones) were studied. All of the osteoclasts counted in this study were mature osteoclasts and had to have met the following criteria: large multinucleated cells, TRAP-positive staining, lying in apposition to a bone surface, and undergoing lacunar resorption (resorption in Howship lacunae) (3, 36). For quantitative image analysis, three parameters were measured: (i) the percentage of bone surface covered by osteoclasts, which is the sum of the length of the osteoclasts containing lacunae (active eroded area) divided by the total length of the suture bone surfaces (this parameter represents the extent of the bone resorption on the suture bone surfaces); (ii) the osteoclast number (ON) (also known as the osteoclast index), which is the number of osteoclasts per millimeter of the suture bone surfaces and reflects changes affecting osteoclast formation; and (iii)the individual osteoclast activity (IOA) (in micrometers per cell), which is the length of lacunar resorption per osteoclast. By measuring the ON and the IOA we could know whether the changes in bone resorption were caused by osteoclastogenesis or by the activity of single osteoclasts. These histomorphometric parameters adhere to the recommended American Society of Bone and Mineral Research nomenclature (22, 23).
All slides were coded by one person and analyzed by another person. The results were verified by a second examiner. Interexaminer and intraexaminer variation was generally less than 10%.
Statistics.
Results are displayed as means ± standard deviations (SD). There were seven mice in each group (n = 7). Analysis of variance was used to analyze differences among groups; in addition, Fisher's one-way analysis of variance and an unpaired two-tailed Student's t test were performed to compare results between treatment groups, and statistical significance was assumed for probability values of ≤0.05.
RESULTS
Note.
The degree of increase was obtained by normalizing an experimental given value with the value from mice of the same strain in the saline-PBS group. Percent bone inhibition was obtained by determining the amount of bone resorption the given treatment prevented from occurring normalized against maximum bone resorption (mice treated with LPS [100 or 500 μg/mouse [each strain]), e.g., (value for LPS-PBS mouse group − value for test group/value for LPS-PBS group × 100).
Percentage of bone surface covered by osteoclasts.
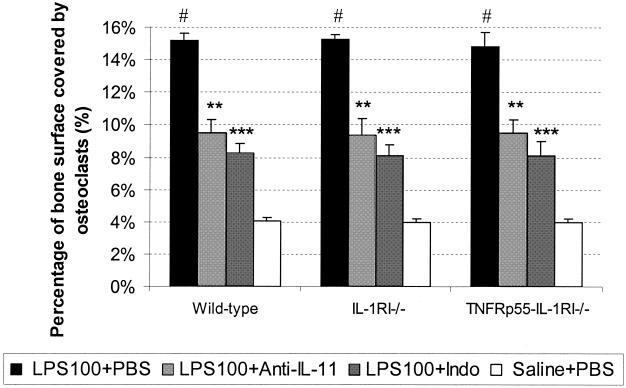
The percentage of bone surface covered by osteoclasts reflects the extent of the bone resorption on the suture bone surfaces. As seen in Fig. 1, low doses of LPS (100 μg/mouse) induced approximately a 3.7-fold increase of this parameter in all three strains of mice relative to values obtained in the saline-PBS group (LPS-PBS versus saline-PBS, 15.2% ± 0.5% versus 4.1% ± 0.2% in wild-type mice and 15.3% ± 0.3% versus 3.9% ± 0.2% in IL-1RI−/− mice; 14.8% ± 0.7% versus 4% ± 0.3% in TNFRp55−/−-IL-1RI−/− mice). In contrast, anti-IL-11 reduced osteoclast coverage by approximately 37% (all P < 0.01), and indomethacin reduced the percentage of bone surface covered by osteoclasts by approximately 46% (all P < 0.001) in all three strains of mice.
FIG. 1.
Percentage of bone surface covered by osteoclasts in mice challenged with a low dose of LPS. All mice received a one-time low dose of LPS (100 μg/mouse) (LPS100) or saline, which was followed by anti-IL-11 MAb (four injections of 465 μg/mouse), indomethacin (Indo) (nine injections of 50 μg/mouse), or PBS injections. Mice were sacrificed on day 5. Bars represent means (error bars, SD), and n was 7 for each group. Symbols: ∗∗ and ∗∗∗, P < 0.01 and P < 0.001, respectively (for comparison with wild-type mice in the LPS100+PBS group); #, P < 0.01 (for comparison with mice of the same strain in the other three groups).
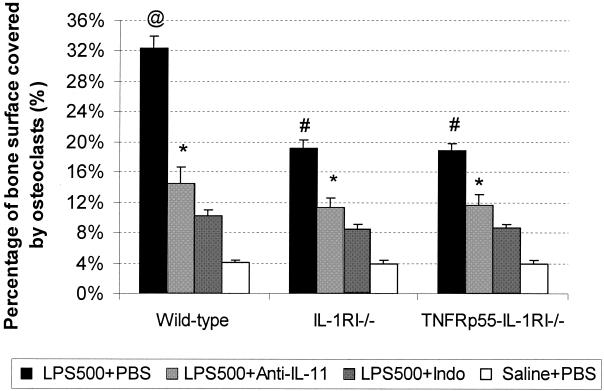
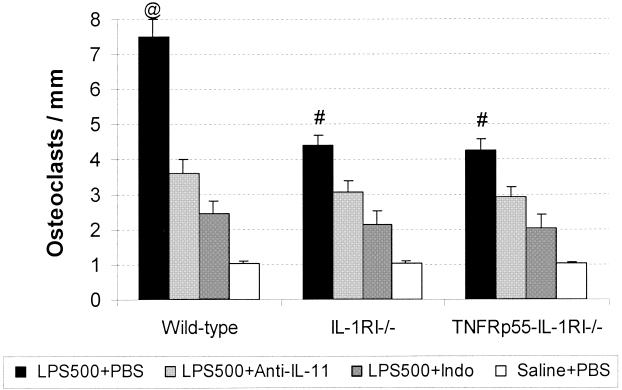
However, at high doses of LPS (500 μg/mouse) (Fig. 2 and 3) an approximately eightfold increase in osteoclast coverage of bone surface in wild-type mice was observed (Fig. 2) (32.4% ± 1.7% in the LPS-PBS group versus 4% ± 0.2% in the saline-PBS group). This is in contrast with an about fivefold increase observed with high-dose LPS challenge in either IL-1RI−/− (19.2% ± 1.0% in the LPS-PBS group versus 4.1% ± 0.2% in the saline-PBS group) or TNFRp55−/−-IL-1RI−/− mice (18.9% ± 0.9% in the LPS-PBS group versus 4.0% ± 0.3% in the saline-PBS group). As shown in Fig. 2, in wild-type mice anti IL-11 antibody reduced bone resorption by 55% (P < 0.001), while indomethacin reduced resorption by 69% (P < 0.001). Bone resorption was inhibited by 50% (P < 0.01) using IL-11 antibody and by 57% (P < 0.01) using indomethacin in both IL-1RI−/− mice and TNFRp55−/−-IL-1RI−/− mice.
FIG. 2.
Percentage of bone surface covered by osteoclasts in mice challenged with a high dose of LPS (500 μg/mouse [LPS500]). The treatments are identical to those described in the legend to Fig. 1. Bars represent means (error bars, SD), and n was 7 for each group. Indo, indomethacin. Symbols: @, P < 0.001 (for comparison with all other groups in this figure); #, P < 0.01 (for comparison with mice of the same strain in the other three groups; ∗, P ≤ 0.05 (for comparison of mice of the same strain in the LPS500+Anti-IL-11 group and the LPS500+Indo group).
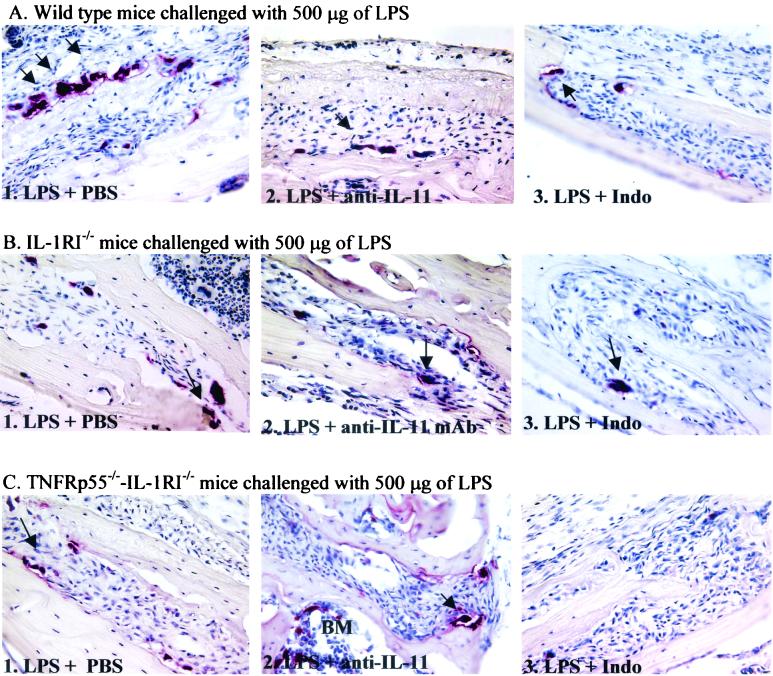
FIG. 3.
Light micrographs of mouse calvaria injected with 500 μg of LPS and treated with either PBS (panels 1), anti-IL-11 MAb (panels 2), or indomethacin (panels 3) in wild-type (A), IL-1RI−/− (B), and TNFRp55−/−-IL-1RI−/− (C) mice. Histological sections of the calvarial bone were stained for TRAP and counterstained with hematoxylin. An arrow indicates TRAP-positive (red-staining) multinucleated cells in Howship's lacunae. Abbreviations: BM, bone marrow; Indo, indomethacin. Magnification, ×200.
ON.
Figure 3 illustrates light micrographs of mouse calvaria injected with 500 μg of LPS and subjected to the various other treatments.
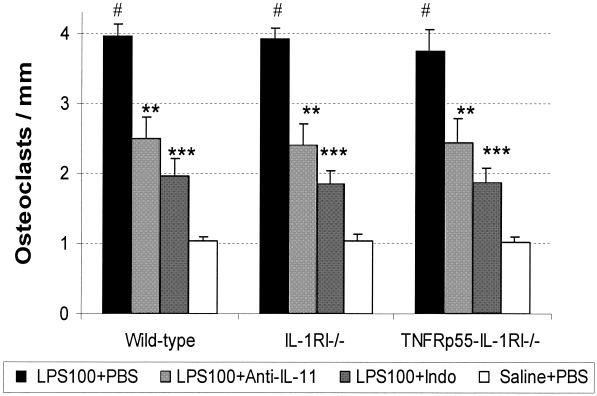
Figure 4 shows that the lower dose of LPS similarly increased the ON by about 3.7-fold (for all results, P < 0.05) in all three strains of mice (4.0 ± 0.2 versus 1.0 ± 0.1 in wild-type mice, 3.9 ± 0.2 versus 1.0 ± 0.1 in IL-1RI−/− mice, 3.7 ± 0.3 versus 1.0 ± 0.1 in TNFRp55−/−-IL-1RI−/− mice). However, the ON was reduced by about 38% with anti-IL-11 MAb (for all results, P < 0.01) and by around 52% with indomethacin (for all results, P < 0.001) in all three strains of mice. At high doses of LPS (Fig. 5), the ON increased by 7.3-fold in wild-type mice (7.5 ± 0.5 versus 1.0 ± 0.1 in wild type) and by 4.2-fold in two strains of knockout mice(4.4 ± 0.3 versus 1.0 ± 0.1 in IL-1RI−/− mice; 4.3 ± 0.3 versus 1.0 ± 0.1 in TNFRp55−/−-IL-1RI−/− mice). However, anti-IL-11 MAb caused a 52% reduction of the ON in wild-type mice and a 30% reduction in two strains of knockouts (for all results, P < 0.01). At high doses of LPS, indomethacin inhibited LPS-induced ON by 69% in wild-type mice and by 51% in IL-1RI−/− and TNFRp55−/−-IL-1RI−/− mice, respectively (for all results, P < 0.001).
FIG. 4.
ON of the calvarial bone surface in mice challenged with a low dose of LPS. All mice received a one-time dose of LPS (100 μg/mouse) (LPS100) or saline, which was followed by anti-IL-11 MAb (four injections of 465 μg/mouse), indomethacin (Indo) (nine injections of 50 μg/mouse), or PBS injections. Mice were sacrificed on day 5. Bars represent means (error bars, SD), and n was 7 for each group. Symbols: ∗∗ and ∗∗∗ P < 0.01 and P < 0.001, respectively (for comparison with wild-type mice in the LPS100+PBS group); #, P < 0.01 (for comparison with mice of the same strain in the other three groups).
FIG. 5.
ON of the calvarial bone surface in mice challenged with a high dose of LPS (500 μg/mouse [LPS500]). The treatments are identical to those described in the legend to Fig. 4. Bars represent means (error bars, SD), and n was 7 for each group. Indo, indomethacin. Symbols: @, P < 0.01 (for comparison with all other groups in this figure); #, P < 0.001 (for comparison with mice of the same strain in the other three groups).
IOA.
The results of IOA indicated that PGs, IL-1, and TNF signaling did not affect the regulation of resorptive activity of single osteoclasts in LPS-induced bone resorption, since no significant differences were found among the three strains of mice with or without indomethacin treatment. However, the IOA (in micrometers per cell) was greater in response to high-dose LPS than to low-dose LPS in all three strains of mice (high-dose LPS-PBS versus low-dose LPS-PBS group: 43.8 ±2.0 versus 36.9 ± 1.5 in wild-type mice, 44.8 ± 3.6 versus 35.7 ± 1.9 in IL-1RI−/− mice, and 42.5 ±2.3 versus 36.5 ±2.2 in TNFRp55−/−-IL-1RI−/− mice [for all results, P < 0.05]), and all LPS-PBS-treated groups had greater IOA values than were seen in saline-PBS controls (saline-PBS group: 32.4 ± 1.1 in wild-type mice, 31.3 ± 1.8 in IL-1−/− mice, and 31.6 ± 1.2 in TNFRp55−/−-IL-1RI−/− mice [for all results, P < 0.05]). IL-11, however, appeared to mediate bone resorption induced by high doses of LPS not only by prompting osteoclastogenesis but also by increasing the resorptive activity of single osteoclasts, since the IOA was lower in high-dose-LPS-anti-IL-11 groups than that in high-dose-LPS-PBS groups in three strains of mice (LPS-anti-IL-11 versus LPS-PBS: 36.5 ± 1.1 versus 43.8 ±2.0 in wild-type, 34.8 ± 1.0 versus 44.8 ± 3.6 in IL-1−/− mice, and 37.0 ±2.0 versus 42.5 ±2.3 in TNFRp55−/−-IL-1RI−/− mice [for all results, P < 0.05]).
DISCUSSION
Since virtually complete inhibition of cytokine activity can be obtained when cytokines or cytokine receptors are genetically deleted from experimental animals, targeted deletions of IL-1 and TNF receptors have been invaluable tools in dissecting the roles of these cytokines in disease processes, particularly in the inflammatory response to LPS (10, 25). In this study, IL-1RI−/− mice and TNFRp55−/−-IL-1RI−/− mice were used.
Although LPS is identified as the major bacterial bone-resorbing factor, surprisingly little is known about its mechanism of action (16). Although several cytokines and local factors, such as IL-1, IL-11, TNF, and PGs, have been reported to stimulate osteoclastogenesis and bone resorption in cell and organ culture systems (9, 14, 16, 21, 36, 39), little is known about their in vivo role and contribution in the regulation of LPS-induced bone resorption. In our previous study, we have suggested two different pathways in LPS-induced bone resorption in vivo (5). The present data substantiate our previous finding (5) that at high doses of LPS, LPS-induced bone resorption is mediated at least in part by IL-1 and TNF receptor signaling, but that this is not true at low LPS doses. Furthermore, the fact that low-dosage-LPS challenge affected all three strains of mice similarly confirmed the fact that in under these conditions LPS-induced bone resorption proceeds through a pathway independent of IL-1 and TNF signaling. The present in vivo study further demonstrates that (i) at low doses of LPS, LPS-induced bone resorption is influenced by IL-11 and PGs, while at high doses it is mediated by IL-11, PGs, and IL-1 receptor signaling; (ii) IL-11 and PGs increase LPS-induced bone resorption by pathways independent of IL-1 or TNF receptor signaling; and (iii) IL-11 and PGs increase LPS-induced bone resorption by enhancing osteoclast formation. In addition, IL-11 was involved in bone resorption induced by high-dose LPS by enhancing the resorptive activity of individual mature osteoclast. The present study provided the relative contribution of each mediator—IL-1, TNF, IL-11, and PGs—in LPS-induced bone resorption in vivo. Since bone resorption was affected similarly in IL-1RI−/− mice and TNFRp55−/−-IL-1RI−/− mice, a minor role of TNF receptor signaling is advocated in LPS-induced bone resorption. However, caution must be exercised in extrapolating these observations to humans given the potential compensatory mechanisms that may exist in IL-1RI−/− and TNFRp55−/−-IL-1RI−/− mice.
Regarding signal transduction pathways, osteoclast formation is induced by at least three different mechanisms. The first mechanism involves the gp130 signal, an important pathway of osteoclast formation, which is activated by cytokines such as IL-11, IL-6, and leukemia inhibitory factor. The second mechanism is the parathyroid hormone-IL-1-PGE axis, which is mediated by signaling involving cyclic AMP. The third mechanism is 1α,25-dihydroxyvitamin D3-induced osteoclast formation, which is mediated by the vitamin D receptor but independent of cyclic AMP (11, 36, 41). In addition, several in vitro studies have found that IL-11 stimulated osteoclast formation in a dose-dependent pattern (9, 11, 29) and was unaffected by inhibitors of IL-1 and TNF (9). Other in vitro studies have also found that LPS stimulated bone resorption by a PGE2-dependent mechanism in mouse bone marrow culture systems (39) and that IL-1 and TNF mediate osteoclastogenesis and bone resorption through PGs, since IL-1α and TNF alpha enhanced osteoclast formation in a dose-responsive manner which was inhibited by indomethacin and reversed by addition of exogenous PGE2 (1, 14). Therefore, IL-11 and PGs may mediate osteoclastogenesis independent of IL-1 and TNF signaling to prompt LPS-induced bone resorption in vivo.
Our findings also indicate that PGs have a potentially stronger activity than IL-11 in LPS-induced bone resorption and osteoclastogenesis in vivo. PGs have been previously found to be essential for osteoclast formation in vitro. Without PGs, osteoclast formation and bone resorption are virtually nonexistent in cell and bone organ culture systems (6, 7, 33). Recent findings demonstrate that IL-11 effects on osteoclast formation were prevented by indomethacin in vitro (9, 11), indicating that, as for IL-1 and TNF (14), the stimulatory effects of IL-11 also involved products of arachidonic acid metabolism (11). Therefore, the stronger ability of indomethacin in reducing bone resorption and osteoclastogenesis may have resulted from inhibition of not only PGs but also other cytokines (such as IL-11 and IL-1) which can mediate their effects through PGs in vivo.
Bone resorption is characterized by numerous sequential events, including osteoclast formation (proliferation, differentiation, and fusion), (pre)osteoclast migration to future resorptive sites, and osteoclast resorptive activity (3, 11, 36). The present study found that anti-IL-11 MAb reduced the IOA in high-dose-LPS groups. This indicates that IL-11 is involved in bone resorption induced by high doses of LPS not only by augmentation of osteoclastogenesis but also in altering the resorptive activity of individual mature osteoclasts. Since IL-11 receptor transcripts were detected in mature osteoclasts, this indicates that mature bone-resorbing cells are potential targets of IL-11 (29). In addition, our results show that IL-11 was involved in mediating the resorptive activity of individual osteoclasts only after high-dose LPS challenge, which suggests that there might be a critical threshold of the sensitivity of osteoclast activity for IL-11. Taken together, these results suggest that IL-11 is an important mediator in bone resorption cascades independent of IL-1 and TNF receptor signaling in LPS-induced inflammatory bone resorption.
Conclusions.
In the present report, an attempt has been made to address the pathway connecting LPS to bone resorption and elucidate the role of IL-11, PGs, IL-1 signaling, and TNF signaling in LPS-induced bone resorption. In addition the relative role of each of these factors in the overall process of bone resorption for groups treated with high and low doses of LPS was determined. At low doses of LPS, osteoclastic bone resorption was caused primarily by enhanced osteoclastogenesis, and this effect was mediated only by IL-11 and PG signaling. These data suggest that in chronic bone diseases involving challenge with low doses of LPS (e.g., periodontal disease), LPS-induced bone resorption results from the effect of IL-11 and PGs on osteoclasts. In contrast, at high doses of LPS, LPS-induced bone resorption appeared to be mediated by a combination of IL-11, PGs, and IL-1 receptor-based signaling. Indomethacin showed slightly stronger activity in reducing LPS-induced osteoclastogenesis and bone resorption than did anti-IL-11 MAb. IL-11, however, appeared to mediate bone resorption induced by high doses of LPS not only by prompting osteoclastogenesis but also by increasing the resorptive activity of single osteoclasts. These data suggest that in acute bone resorption processes involving high doses of LPS (e.g., periodontal abscesses) LPS-induced bone resorption results from the combinatorial effects of IL-1, IL-11, and PGs.
Acknowledgments
We are indebted to J. Peschon at Immunex Corp. for the generous gift of the transgenic IL-1RI−/− and TNFRp55−/−/IL-1RI−/− mice and to Genetics Institute, Inc., for anti IL-11 MAb.
This study was supported by National Institutes of Health grants 12482 (to S. Amar) and DE07559 (to D. T. Graves).
Editor: R. N. Moore
REFERENCES
- 1.Akatsu, T., N. Takahashi, N. Udagawa, K. Imamura, A. Yamaguchi, K. Sato, N. Nagata, and T. Suda. 1991. Role of prostaglandins in interleukin-1-induced bone resorption in mice in vitro. J. Bone Miner. Res. 6:183-189. [DOI] [PubMed] [Google Scholar]
- 2.Arend, W. P., M. Malyak, M. F. Smith, Jr., T. D. Whisenand, J. L. Slack, J. E. Sims, J. G. Giri, and S. K. Dower. 1994. Binding of IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist by soluble IL-1 receptors and levels of soluble IL-1 receptors in synovial fluids. J. Immunol. 153:4766-4774. [PubMed] [Google Scholar]
- 3.Athanasou, N. A. 1996. Cellular biology of bone-resorbing cells. J. Bone Joint Surg. Am. 78:1096-1112. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, B. F., T. B. Aufdemorte, I. R. Garrett, A. J. Yates, and G. R. Mundy. 1989. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology 125:1142-1150. [DOI] [PubMed] [Google Scholar]
- 5.Chiang, C. Y., G. Kyritsis, D. T. Graves, and S. Amar. 1999. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 67:4231-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, D. A., and T. J. Chambers. 1991. Effect of prostaglandins E1, E2, and F2 alpha on osteoclast formation in mouse bone marrow cultures. J. Bone Miner. Res. 6:157-164. [DOI] [PubMed] [Google Scholar]
- 7.Collins, D. A., and T. J. Chambers. 1992. Prostaglandin E2 promotes osteoclast formation in murine hematopoietic cultures through an action on hematopoietic cells. J. Bone Miner. Res. 7:555-561. [DOI] [PubMed] [Google Scholar]
- 8.Elias, J. A., W. Tang, and M. C. Horowitz. 1995. Cytokine and hormonal stimulation of human osteosarcoma interleukin-11 production. Endocrinology 136:489-498. [DOI] [PubMed] [Google Scholar]
- 9.Girasole, G., G. Passeri, R. L. Jilka, and S. C. Manolagas. 1994. Interleukin-11: a new cytokine critical for osteoclast development. J. Clin. Investig. 93:1516-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glaccum, M. B., K. L. Stocking, K. Charrier, J. L. Smith, C. R. Willis, C. Maliszewski, D. J. Livingston, J. J. Peschon, and P. J. Morrissey. 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 159:3364-3371. [PubMed] [Google Scholar]
- 11.Hill, P. A., A. Tumber, S. Papaioannou, and M. C. Meikle. 1998. The cellular actions of interleukin-11 on bone resorption in vitro. Endocrinology 139:1564-1572. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto, T., S. Akira, M. Narazaki, and T. Taga. 1995. Interleukin-6 family of cytokines and gp130. Blood 86:1243-1254. [PubMed] [Google Scholar]
- 13.Kornman, K. S. 1999. Host modulation as a therapeutic strategy in the treatment of periodontal disease. Clin. Infect. Dis. 28:520-526. [DOI] [PubMed] [Google Scholar]
- 14.Lader, C. S., and A. M. Flanagan. 1998. Prostaglandin E2, interleukin 1alpha, and tumor necrosis factor-alpha increase human osteoclast formation and bone resorption in vitro. Endocrinology 139:3157-3164. [DOI] [PubMed] [Google Scholar]
- 15.Maier, R., V. Ganu, and M. Lotz. 1993. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J. Biol. Chem. 268:21527-21532. [PubMed] [Google Scholar]
- 16.Nair, S. P., S. Meghji, M. Wilson, K. Reddi, P. White, and B. Henderson. 1996. Bacterially induced bone destruction: mechanisms and misconceptions. Infect. Immun. 64:2371-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhaus, H., B. Bettenhausen, P. Bilinski, D. Simon-Chazottes, J. L. Guenet, and A. Gossler. 1994. Etl2, a novel putative type-I cytokine receptor expressed during mouse embryogenesis at high levels in skin and cells with skeletogenic potential. Dev. Biol. 166:531-542. [DOI] [PubMed] [Google Scholar]
- 18.Offenbacher, S. 1996. Periodontal diseases: pathogenesis. Ann. Periodontol. 1:821-878. [DOI] [PubMed] [Google Scholar]
- 19.Offenbacher, S., B. M. Odle, and T. E. Van Dyke. 1986. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J. Periodontal Res. 21:101-112. [DOI] [PubMed] [Google Scholar]
- 20.Offenbacher, S., and G. E. Salvi. 1999. Induction of prostaglandin release from macrophages by bacterial endotoxin. Clin. Infect. Dis. 28:505-513. [DOI] [PubMed] [Google Scholar]
- 21.Ohlin, A., U. Sjogren, and U. H. Lerner. 1999. Bone resorbing activity released from zymosan-activated mouse peritoneal macrophages-the role of prostanoids and interleukin-1. Inflamm. Res. 48:181-192. [DOI] [PubMed] [Google Scholar]
- 22.Parfitt, A. M. 1988. Bone histomorphometry: proposed system for standardization of nomenclature, symbols, and units. Calcif. Tissue Int. 42:284-286. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt, A. M., M. K. Drezner, F. H. Glorieux, J. A. Kanis, H. Malluche, P. J. Meunier, S. M. Ott, and R. R. Recker. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2:595-610. [DOI] [PubMed] [Google Scholar]
- 24.Paul, S. R., F. Bennett, J. A. Calvetti, K. Kelleher, C. R. Wood, R. M. O'Hara, Jr., A. C. Leary, B. Sibley, S. C. Clark, D. A. Williams, et al. 1990. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc. Natl. Acad. Sci. USA 87:7512-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peschon, J. J., D. S. Torrance, K. L. Stocking, M. B. Glaccum, C. Otten, C. R. Willis, K. Charrier, P. J. Morrissey, C. B. Ware, and K. M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160:943-952. [PubMed] [Google Scholar]
- 26.Raisz, L. 1984. Prostaglandins in bone and mineral metabolism, 2nd ed., vol. 2. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 27.Reddi, K., S. Poole, S. Nair, S. Meghji, B. Henderson, and M. Wilson. 1995. Lipid A-associated proteins from periodontopathogenic bacteria induce interleukin-6 production by human gingival fibroblasts and monocytes. FEMS Immunol. Med. Microbiol. 11:137-144. [DOI] [PubMed] [Google Scholar]
- 28.Rietschel, E. T., and H. Brade. 1992. Bacterial endotoxins. Sci. Am. 267:54-61. [DOI] [PubMed] [Google Scholar]
- 29.Romas, E., N. Udagawa, H. Zhou, T. Tamura, M. Saito, T. Taga, D. J. Hilton, T. Suda, K. W. Ng, and T. J. Martin. 1996. The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J. Exp. Med. 183:2581-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 31.Ruby, J., H. Bluethmann, and J. J. Peschon. 1997. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J. Exp. Med. 186:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheridan, B. C., C. A. Dinarello, D. R. Meldrum, D. A. Fullerton, C. H. Selzman, and R. C. McIntyre, Jr. 1999. Interleukin-11 attenuates pulmonary inflammation and vasomotor dysfunction in endotoxin-induced lung injury. Am. J. Physiol. 277:L861-L867. [DOI] [PubMed] [Google Scholar]
- 33.Shinar, D. M., and G. A. Rodan. 1990. Biphasic effects of transforming growth factor-beta on the production of osteoclast-like cells in mouse bone marrow cultures: the role of prostaglandins in the generation of these cells. Endocrinology 126:3153-3158. [DOI] [PubMed] [Google Scholar]
- 34.Sims, J. E., M. A. Gayle, J. L. Slack, M. R. Alderson, T. A. Bird, J. G. Giri, F. Colotta, F. Re, A. Mantovani, K. Shanebeck, et al. 1993. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc. Natl. Acad. Sci. USA 90:6155-6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sismey-Durrant, H. J., and R. M. Hopps. 1991. Effect of lipopolysaccharide from Porphyromonas gingivalis on prostaglandin E2 and interleukin-1-beta release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol. Immunol. 6:378-380. [DOI] [PubMed] [Google Scholar]
- 36.Suda, T., N. Takahashi, N. Udagawa, E. Jimi, M. T. Gillespie, and T. J. Martin. 1999. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20:345-357. [DOI] [PubMed] [Google Scholar]
- 37.Taubman, M. A., and T. Kawai. 2001. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit. Rev. Oral Biol. Med. 12:125-135. [DOI] [PubMed] [Google Scholar]
- 38.Tobias, P. S., J. Gegner, R. Tapping, S. Orr, J. Mathison, J. D. Lee, V. Kravchenko, J. Han, and R. J. Ulevitch. 1997. Lipopolysaccharide dependent cellular activation. J. Periodontal Res. 32:99-103. [DOI] [PubMed] [Google Scholar]
- 39.Ueda, N., M. Koide, M. Ohguchi, Y. Ishihara, T. Noguchi, N. Okahashi, and T. Nishihara. 1998. Involvement of prostaglandin E2 and interleukin-1 alpha in the differentiation and survival of osteoclasts induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4. J. Periodontal Res. 33:509-516. [DOI] [PubMed] [Google Scholar]
- 40.Williams, R. C. 1999. Non-steroidal anti-inflammatory drugs for altering periodontal bone loss. J. Dent. Res. 78:638-642. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, M. Goto, S. I. Mochizuki, E. Tsuda, T. Morinaga, N. Udagawa, N. Takahashi, T. Suda, and K. Higashio. 1999. A novel molecular mechanism modulating osteoclast differentiation and function. Bone 25:109-113. [DOI] [PubMed] [Google Scholar]