Abstract
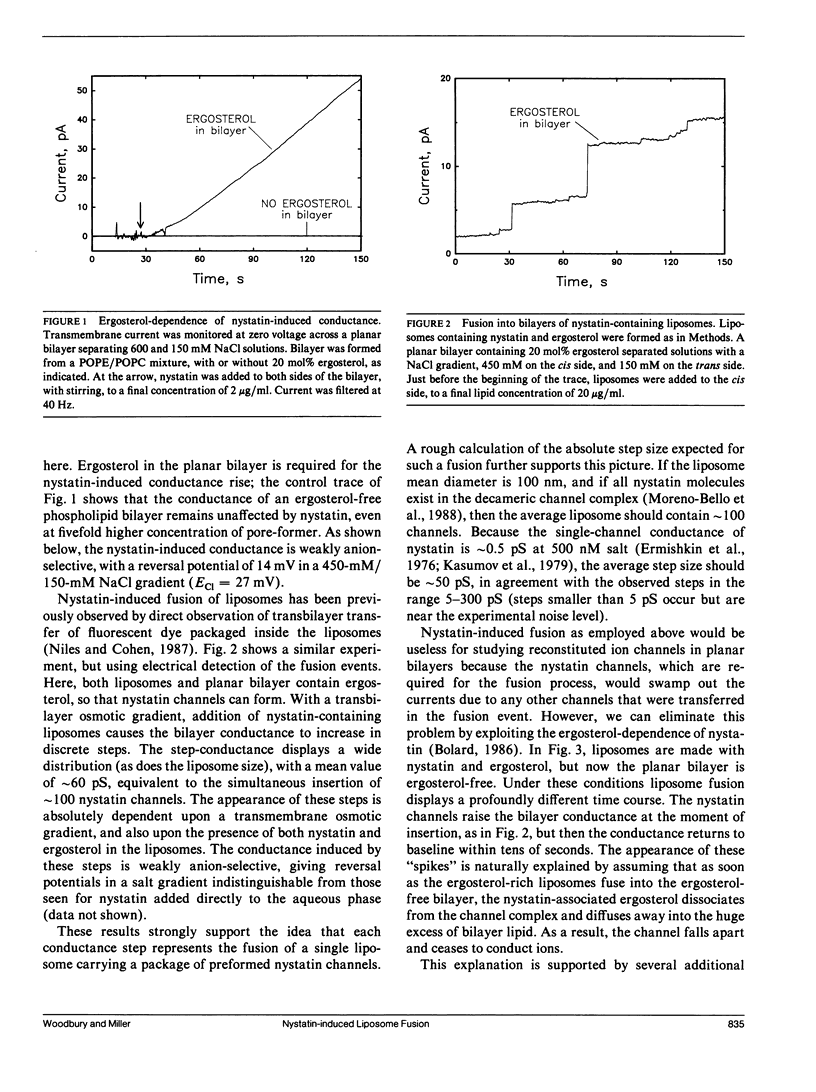
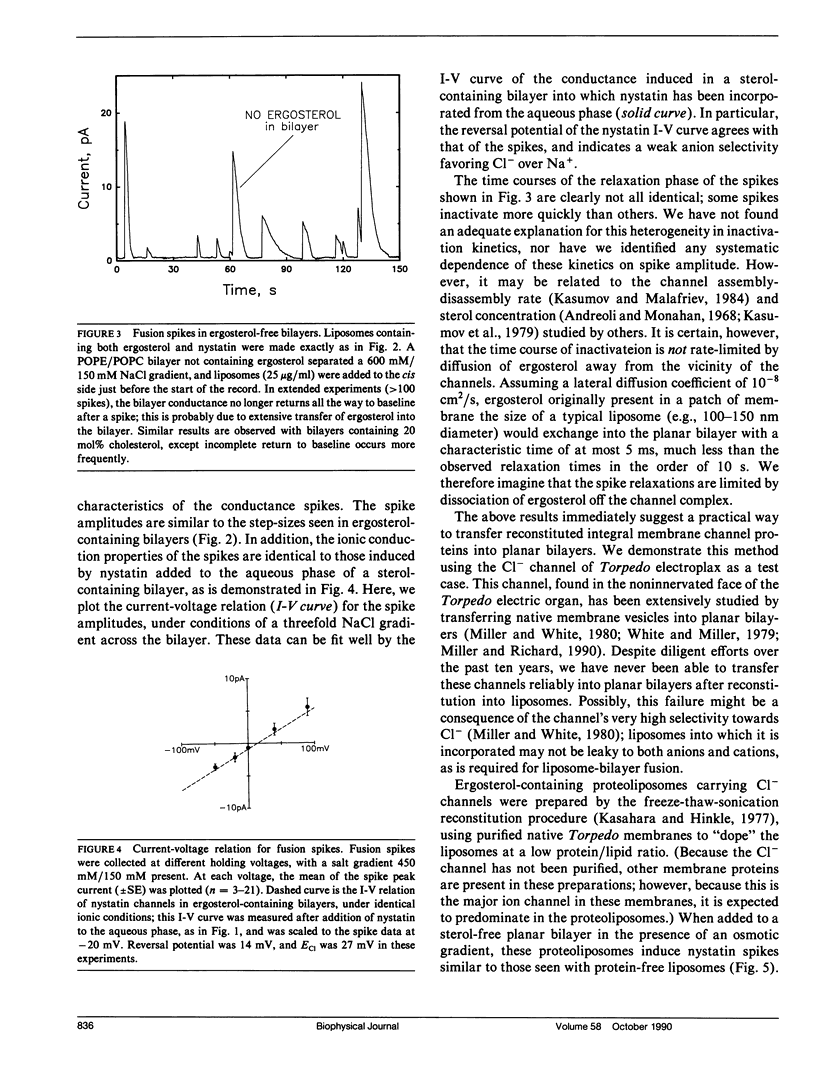
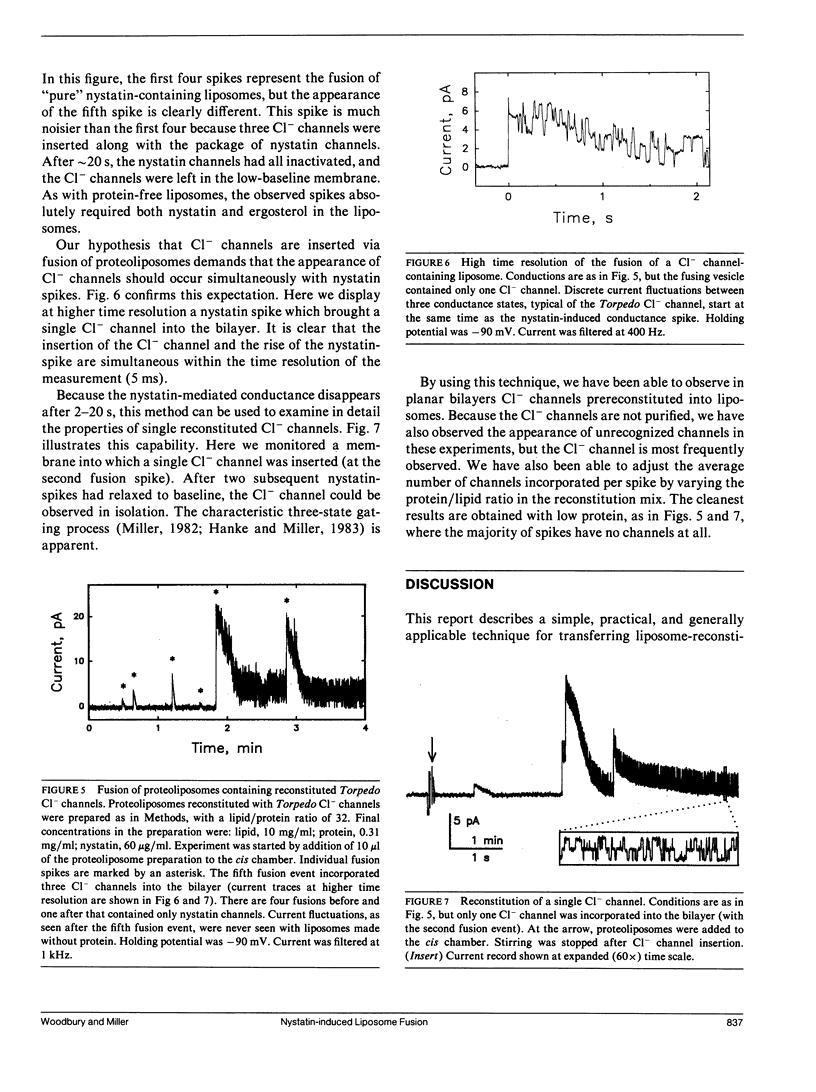
A simple method is described for promoting and detecting fusion of liposomes with planar bilayer membranes. Liposomes containing ergosterol are doped with the pore-forming antibiotic nystatin, and the planar bilayer is kept ergosterol-free. Under these conditions, when a transbilayer salt gradient is applied, liposomes added to the high-salt side of the bilayer elicit the appearance of abrupt conductance jumps of 5-300 pS. The increase in conductance is transient, decaying back to baseline on the order of 10 s. Each of these "spikes" represents the fusion of a single liposome with the bilayer, resulting in the simultaneous insertion of many nystatin channels. Relaxation of the conductance back to baseline occurs because ergosterol, required for the integrity of the nystatin pore, diffuses away into the sterol-free planar bilayer after liposome fusion. When Torpedo Cl- channels are reconstituted into liposomes containing ergosterol and nystatin, fusion spikes are observed simultaneously with the appearance of Cl- channels. This method allows the calculation of the density of functional ion channels in a preparation of proteoliposomes containing reconstituted channel protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Monahan M. The interaction of polyene antibiotics with thin lipid membranes. J Gen Physiol. 1968 Aug;52(2):300–325. doi: 10.1085/jgp.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Cass A., Finkelstein A., Krespi V. The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970 Jul;56(1):100–124. doi: 10.1085/jgp.56.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. S., Niles W. D., Akabas M. H. Fusion of phospholipid vesicles with a planar membrane depends on the membrane permeability of the solute used to create the osmotic pressure. J Gen Physiol. 1989 Feb;93(2):201–210. doi: 10.1085/jgp.93.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. S., Zimmerberg J., Finkelstein A. Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. II. Incorporation of a vesicular membrane marker into the planar membrane. J Gen Physiol. 1980 Mar;75(3):251–270. doi: 10.1085/jgp.75.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermishkin L. N., Kasumov K. M., Potzeluyev V. M. Single ionic channels induced in lipid bilayers by polyene antibiotics amphotericin B and nystatine. Nature. 1976 Aug 19;262(5570):698–699. doi: 10.1038/262698a0. [DOI] [PubMed] [Google Scholar]
- Hanke W., Miller C. Single chloride channels from Torpedo electroplax. Activation by protons. J Gen Physiol. 1983 Jul;82(1):25–45. doi: 10.1085/jgp.82.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kasumov K. M., Borisova M. P., Ermishkin L. N., Potseluyev V. M., Silberstein A. Y., Vainshtein V. A. How do ionic channel properties depend on the structure of polyene antibiotic molecules? Biochim Biophys Acta. 1979 Mar 8;551(2):229–237. doi: 10.1016/0005-2736(89)90001-1. [DOI] [PubMed] [Google Scholar]
- Miller C., Arvan P., Telford J. N., Racker E. Ca++-induced fusion of proteoliposomes: dependence on transmembrane osmotic gradient. J Membr Biol. 1976;30(3):271–282. doi: 10.1007/BF01869672. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Miller C., White M. M. A voltage-dependent chloride conductance channel from Torpedo electroplax membrane. Ann N Y Acad Sci. 1980;341:534–551. doi: 10.1111/j.1749-6632.1980.tb47197.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Bello M., Bonilla-Marín M., González-Beltrán C. Distribution of pore sizes in black lipid membranes treated with nystatin. Biochim Biophys Acta. 1988 Sep 15;944(1):97–100. doi: 10.1016/0005-2736(88)90321-5. [DOI] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S., Finkelstein A. Hydrostatic pressures developed by osmotically swelling vesicles bound to planar membranes. J Gen Physiol. 1989 Feb;93(2):211–244. doi: 10.1085/jgp.93.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles W. D., Cohen F. S. Video fluorescence microscopy studies of phospholipid vesicle fusion with a planar phospholipid membrane. Nature of membrane-membrane interactions and detection of release of contents. J Gen Physiol. 1987 Nov;90(5):703–735. doi: 10.1085/jgp.90.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch Biochem Biophys. 1981 Nov;212(1):186–194. doi: 10.1016/0003-9861(81)90358-1. [DOI] [PubMed] [Google Scholar]
- White M. M., Miller C. A voltage-gated anion channel from the electric organ of Torpedo californica. J Biol Chem. 1979 Oct 25;254(20):10161–10166. [PubMed] [Google Scholar]
- Woodbury D. J., Hall J. E. Role of channels in the fusion of vesicles with a planar bilayer. Biophys J. 1988 Dec;54(6):1053–1063. doi: 10.1016/S0006-3495(88)83042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D. J., Hall J. E. Vesicle-membrane fusion. Observation of simultaneous membrane incorporation and content release. Biophys J. 1988 Aug;54(2):345–349. doi: 10.1016/S0006-3495(88)82965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


