Abstract
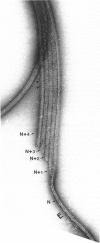
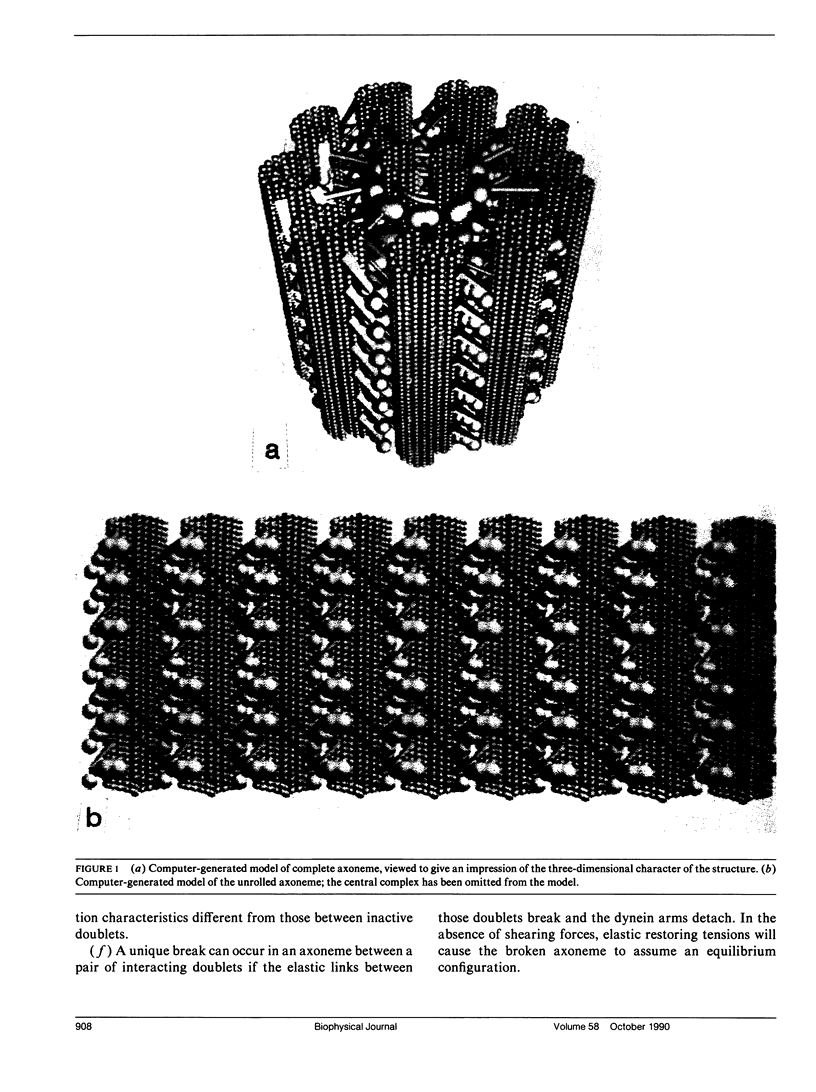
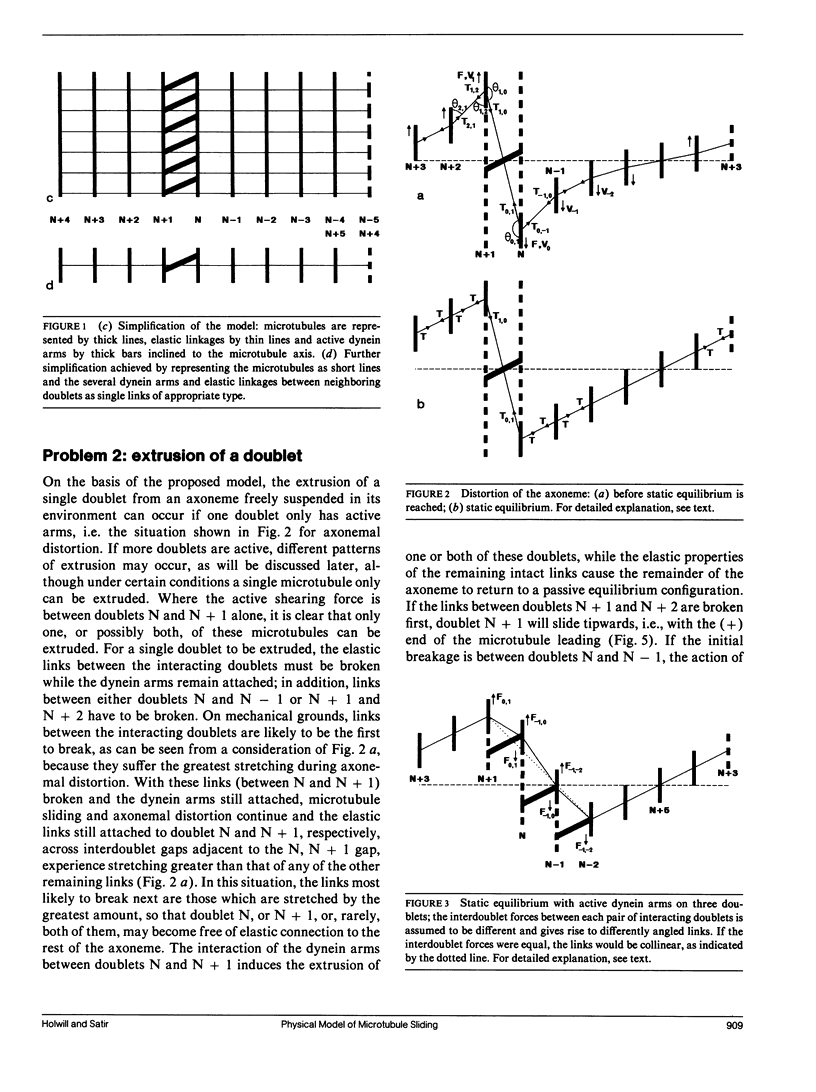
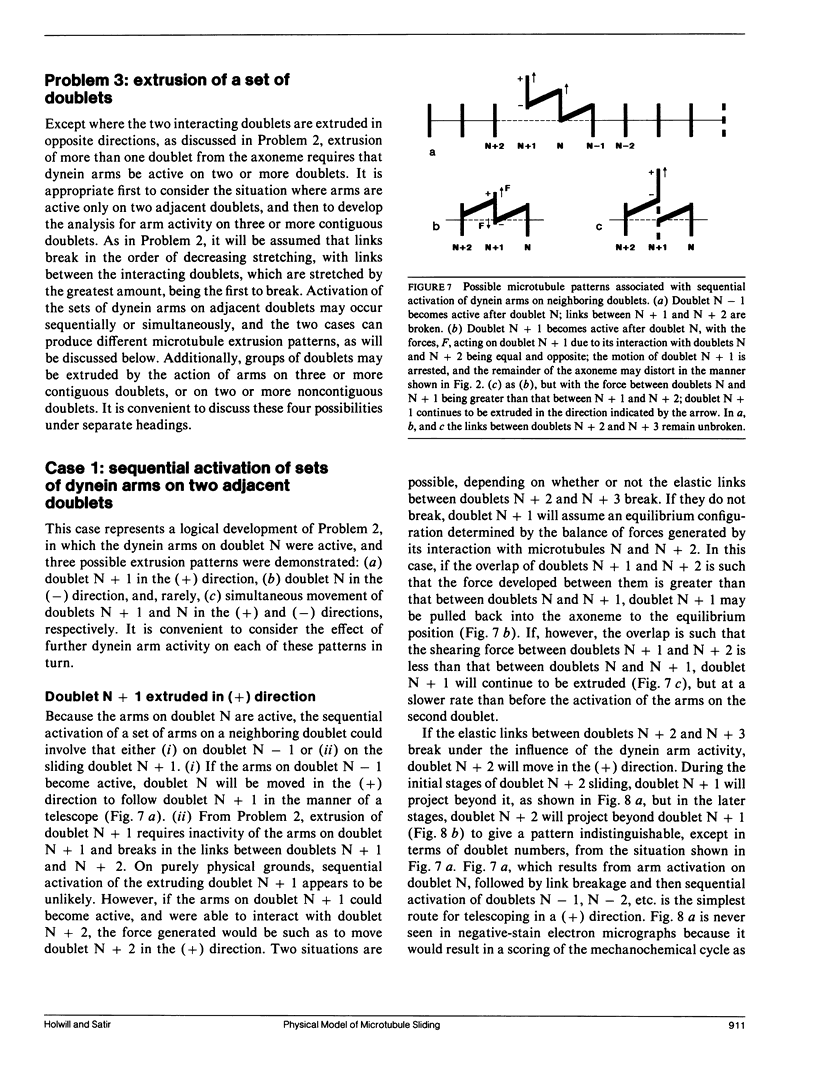
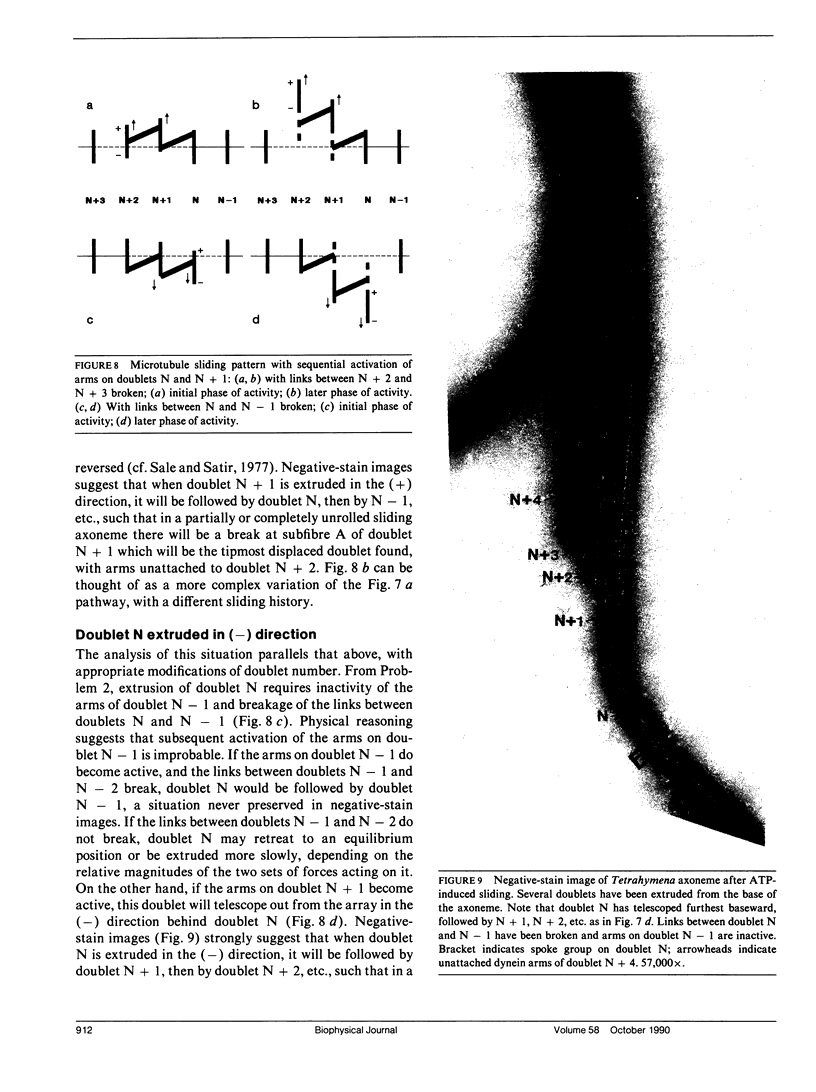
Ciliary movement is caused by coordinated sliding interactions between the peripheral doublet microtubules of the axoneme. In demembranated organelles treated with trypsin and ATP, this sliding can be visualized during progressive disintegration. In this paper, microtubule sliding behavior resulting from various patterns of dynein arm activity and elastic link breakage is determined using a simplified model of the axoneme. The model consists of a cylindrical array of microtubules joined, initially, by elastic links, with the possibility of dynein arm interaction between microtubules. If no elastic links are broken, sliding can produce stable distortion of the model, which finds application to straight sections of a motile cilium. If some elastic links break, the model predicts a variety of sliding patterns, some of which match, qualitatively, the observed disintegration behavior of real axonemes. Splitting of the axoneme is most likely to occur between two doublets N and N + 1 when either the arms on doublet N + 1 are active and arms on doublet N are inactive or arms on doublet N - 1 are active while arms on doublet N are inactive. The analysis suggests further experimental studies which, in conjunction with the model, will lead to a more detailed understanding of the sliding mechanism, and will allow the mechanical properties of some axonemal components to be evaluated.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avolio J., Glazzard A. N., Holwill M. E., Satir P. Structures attached to doublet microtubules of cilia: computer modeling of thin-section and negative-stain stereo images. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4804–4808. doi: 10.1073/pnas.83.13.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- Sale W. S., Satir P. Direction of active sliding of microtubules in Tetrahymena cilia. Proc Natl Acad Sci U S A. 1977 May;74(5):2045–2049. doi: 10.1073/pnas.74.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale W. S., Satir P. Splayed Tetrahymena cilia. A system for analyzing sliding and axonemal spoke arrangements. J Cell Biol. 1976 Nov;71(2):589–605. doi: 10.1083/jcb.71.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale W. S. The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol. 1986 Jun;102(6):2042–2052. doi: 10.1083/jcb.102.6.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P., Matsuoka T. Splitting the ciliary axoneme: implications for a "switch-point" model of dynein arm activity in ciliary motion. Cell Motil Cytoskeleton. 1989;14(3):345–358. doi: 10.1002/cm.970140305. [DOI] [PubMed] [Google Scholar]
- Satir P. Studies on cilia. 3. Further studies on the cilium tip and a "sliding filament" model of ciliary motility. J Cell Biol. 1968 Oct;39(1):77–94. doi: 10.1083/jcb.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P., Wais-Steider J., Lebduska S., Nasr A., Avolio J. The mechanochemical cycle of the dynein arm. Cell Motil. 1981;1(3):303–327. doi: 10.1002/cm.970010304. [DOI] [PubMed] [Google Scholar]
- Sato F., Mogami Y., Baba S. A. Flagellar quiescence and transience of inactivation induced by rapid pH drop. Cell Motil Cytoskeleton. 1988;10(3):374–379. doi: 10.1002/cm.970100304. [DOI] [PubMed] [Google Scholar]
- Spungin B., Avolio J., Arden S., Satir P. Dynein arm attachment probed with a non-hydrolyzable ATP analog. Structural evidence for patterns of activity. J Mol Biol. 1987 Oct 20;197(4):671–677. doi: 10.1016/0022-2836(87)90473-6. [DOI] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Effects of trypsin digestion on flagellar structures and their relationship to motility. J Cell Biol. 1973 Sep;58(3):618–629. doi: 10.1083/jcb.58.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Shingyoji C., Kamimura S. Microtubule sliding in reactivated flagella. Symp Soc Exp Biol. 1982;35:159–177. [PubMed] [Google Scholar]
- Warner F. D. Ciliary inter-microtubule bridges. J Cell Sci. 1976 Jan;20(1):101–114. doi: 10.1242/jcs.20.1.101. [DOI] [PubMed] [Google Scholar]
- Woolley D. M., Brammall A. Direction of sliding and relative sliding velocities within trypsinized sperm axonemes of Gallus domesticus. J Cell Sci. 1987 Oct;88(Pt 3):361–371. doi: 10.1242/jcs.88.3.361. [DOI] [PubMed] [Google Scholar]
- Zanetti N. C., Warner F. D. Evidence for a role of 13S axonemal ATPase in modulation of ciliary microtubule sliding. Cell Motil. 1982;2(6):509–523. doi: 10.1002/cm.970020603. [DOI] [PubMed] [Google Scholar]