Abstract
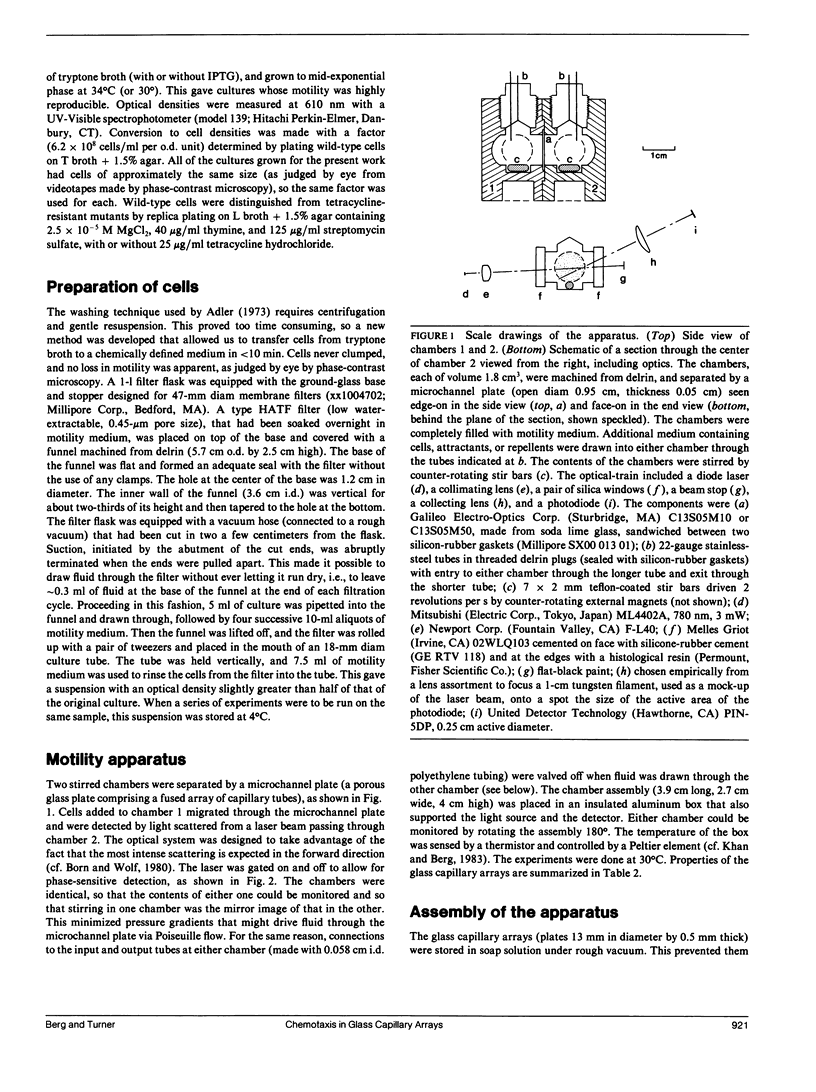
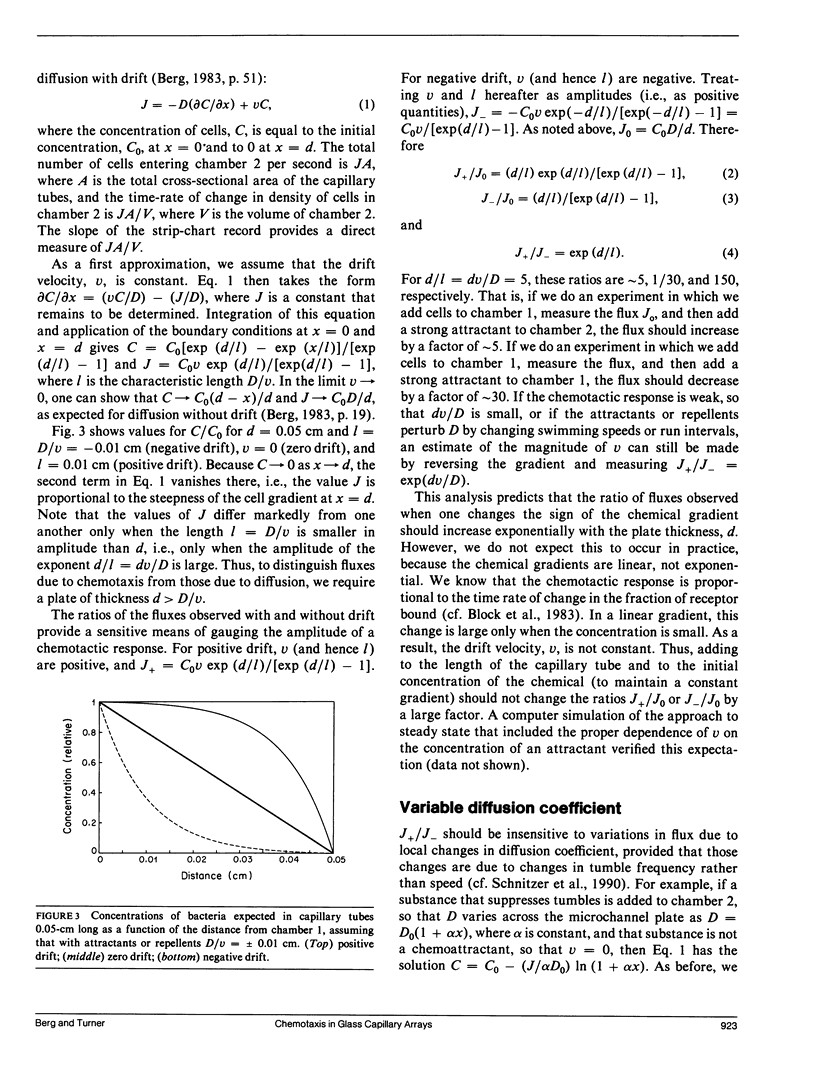
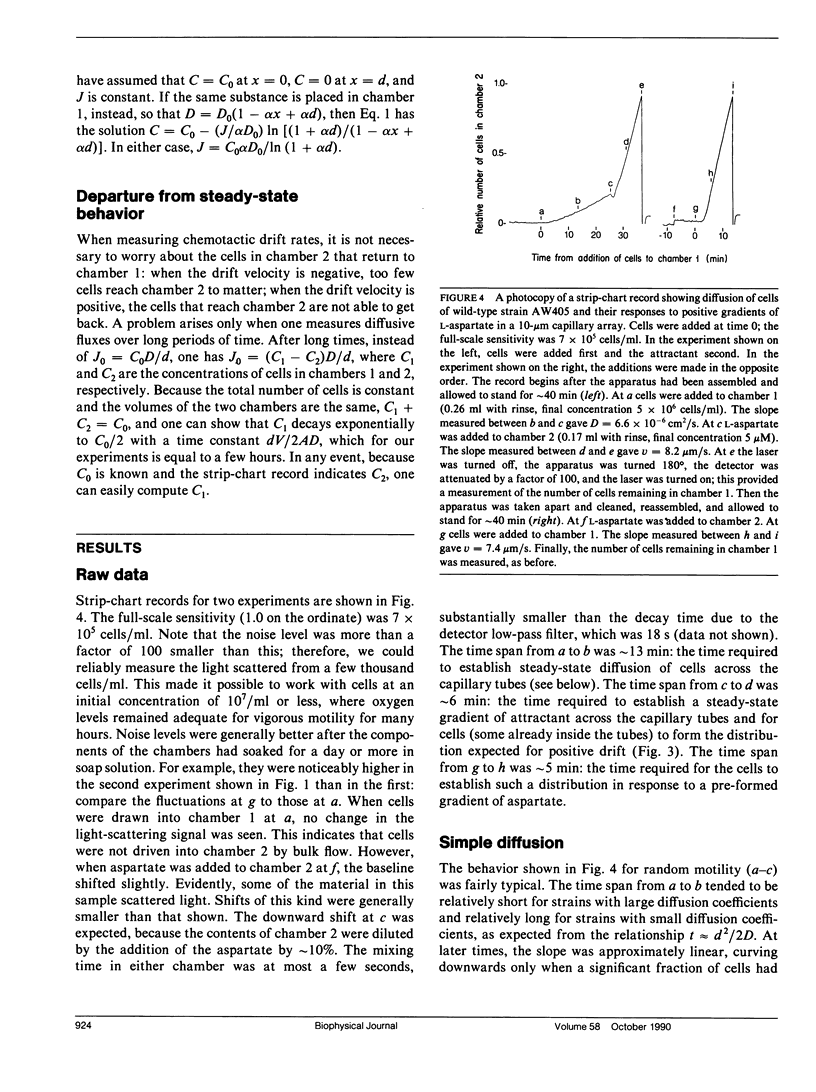
Random and directed motility of bacterial populations were assayed by monitoring the flux of bacteria through a microchannel plate (a porous glass plate comprising a fused array of capillary tubes) separating two identical stirred chambers. Cells, washed free of growth medium by a new filtration method, were added to one chamber at a low density. Their number in the other chamber was determined from the amount of light scattered from a beam of a laser diode and recorded on a strip chart. Diffusion coefficients were computed from fluxes observed in the absence of chemical gradients, and chemotaxis drift velocities were computed from fluxes observed in their presence. Cells migrated through tubes of diam 10 microns more rapidly than through tubes of diam 50 microns, suggesting that the straight segments of their tracks were aligned with the axes of the smaller tubes. Mutants that are motile but nonchemotactic could be selected because they move through the microchannel plate in the face of an adverse gradient. Weak chemotactic responses were assessed from ratios of fluxes observed in paired experiments in which the sign of the gradient of attractant was reversed. Studies were made of wild-type Escherichia coli and mutants that are nonmotile, tumblely, smooth-swimming, aspartate-blind, or defective in methylation and demethylation. Chemotaxis drift velocities for the latter mutants (cheRcheB) were quite small.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Berg H. C. A physicist looks at bacterial chemotaxis. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):1–9. doi: 10.1101/sqb.1988.053.01.003. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Block S. M. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984 Nov;130(11):2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. How to track bacteria. Rev Sci Instrum. 1971 Jun;42(6):868–871. doi: 10.1063/1.1685246. [DOI] [PubMed] [Google Scholar]
- Block S. M., Berg H. C. Successive incorporation of force-generating units in the bacterial rotary motor. 1984 May 31-Jun 6Nature. 309(5967):470–472. doi: 10.1038/309470a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Segall J. E., Berg H. C. Adaptation kinetics in bacterial chemotaxis. J Bacteriol. 1983 Apr;154(1):312–323. doi: 10.1128/jb.154.1.312-323.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret R. B., Hess J. F., Borkovich K. A., Pakula A. A., Simon M. I. Protein phosphorylation in chemotaxis and two-component regulatory systems of bacteria. J Biol Chem. 1989 May 5;264(13):7085–7088. [PubMed] [Google Scholar]
- Chang Y. Y., Cronan J. E., Jr Mapping nonselectable genes of Escherichia coli by using transposon Tn10: location of a gene affecting pyruvate oxidase. J Bacteriol. 1982 Sep;151(3):1279–1289. doi: 10.1128/jb.151.3.1279-1289.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Koshland D. E., Jr Membrane receptors for aspartate and serine in bacterial chemotaxis. J Biol Chem. 1979 Oct 10;254(19):9695–9702. [PubMed] [Google Scholar]
- Dahlquist F. W., Elwell R. A., Lovely P. S. Studies of bacterial chemotaxis in defined concentration gradients. A model for chemotaxis toward L-serine. J Supramol Struct. 1976;4(3):329–342. doi: 10.1002/jss.400040304. [DOI] [PubMed] [Google Scholar]
- Dahlquist F. W., Lovely P., Koshland D. E., Jr Quantitative analysis of bacterial migration in chemotaxis. Nat New Biol. 1972 Mar 29;236(65):120–123. doi: 10.1038/newbio236120a0. [DOI] [PubMed] [Google Scholar]
- Khan S., Berg H. C. Isotope and thermal effects in chemiosmotic coupling to the flagellar motor of Streptococcus. Cell. 1983 Mar;32(3):913–919. doi: 10.1016/0092-8674(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W., Vogler A. P. Molecular mechanisms of bacterial chemotaxis towards PTS-carbohydrates. FEMS Microbiol Rev. 1989 Jun;5(1-2):81–92. doi: 10.1016/0168-6445(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., DeRosier D. J. Bacterial flagellar structure and function. Can J Microbiol. 1988 Apr;34(4):442–451. doi: 10.1139/m88-077. [DOI] [PubMed] [Google Scholar]
- Nossal R. Growth and movement of rings of chemotactic bacteria. Exp Cell Res. 1972 Nov;75(1):138–142. doi: 10.1016/0014-4827(72)90529-0. [DOI] [PubMed] [Google Scholar]
- Nossal R., Weiss G. H. Analysis of a densitometry assay for bacterial chemotaxis. J Theor Biol. 1973 Sep 14;41(1):143–147. doi: 10.1016/0022-5193(73)90194-x. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Doing behavioral genetics with bacteria. Genetics. 1987 Aug;116(4):499–500. doi: 10.1093/genetics/116.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J. E., Block S. M., Berg H. C. Temporal comparisons in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel L. A., Chet I., Henis Y. A simple quantitative assay for bacterial motility. J Gen Microbiol. 1977 Feb;98(2):329–337. doi: 10.1099/00221287-98-2-329. [DOI] [PubMed] [Google Scholar]
- Segel L. A., Jackson J. L. Theoretical analysis of chemotactic movement in bacteria. J Mechanochem Cell Motil. 1973 May;2(1):25–34. [PubMed] [Google Scholar]
- Sherris D., Parkinson J. S. Posttranslational processing of methyl-accepting chemotaxis proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6051–6055. doi: 10.1073/pnas.78.10.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Stock J., Borczuk A., Chiou F., Burchenal J. E. Compensatory mutations in receptor function: a reevaluation of the role of methylation in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8364–8368. doi: 10.1073/pnas.82.24.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis R. M., Koshland D. E., Jr Reversible receptor methylation is essential for normal chemotaxis of Escherichia coli in gradients of aspartic acid. Proc Natl Acad Sci U S A. 1988 Jan;85(1):83–87. doi: 10.1073/pnas.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Berg H. C. Migration of bacteria in semisolid agar. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6973–6977. doi: 10.1073/pnas.86.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Berg H. C. Acetyladenylate plays a role in controlling the direction of flagellar rotation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6711–6715. doi: 10.1073/pnas.85.18.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Kramer T. J., Berg H. C. Reconstitution of signaling in bacterial chemotaxis. J Bacteriol. 1987 May;169(5):1878–1885. doi: 10.1128/jb.169.5.1878-1885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


