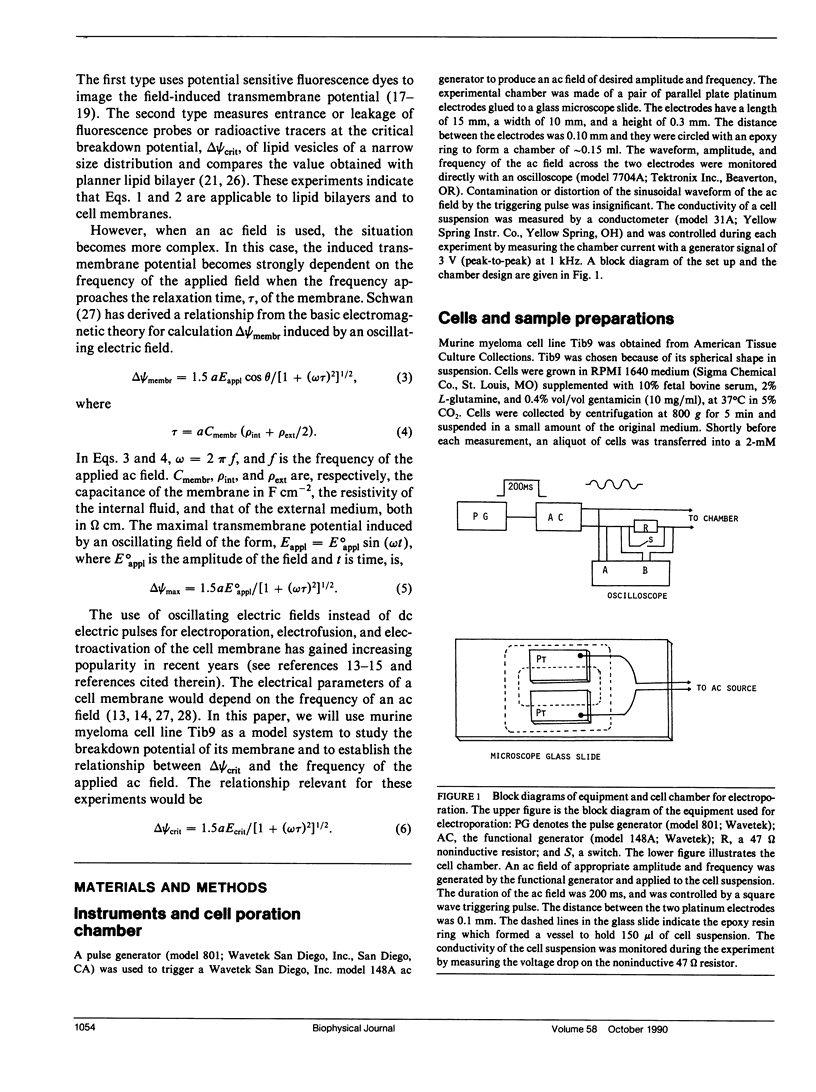
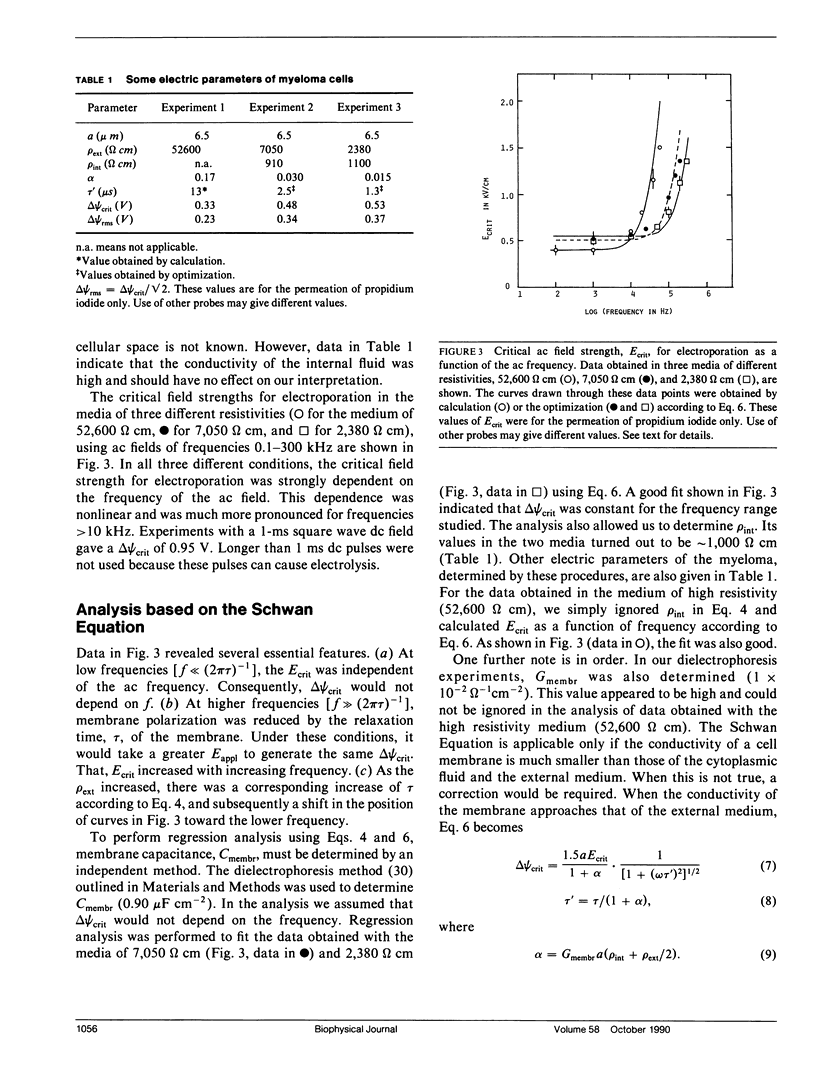
Abstract
The transmembrane potential generated by an alternating electric field (ac) depends strongly on the frequency of the field and can be calculated using the Schwan Equation. We have measured the critical electric breakdown potential, delta psi crit, of the plasma membrane of murine myeloma cell line (Tib9) using ac fields, by monitoring the entry of a fluorescence probe, propidium iodide, into the cells. This dye is weakly fluorescent in solution but becomes strongly fluorescent when it binds to DNA. Experiments were done under a microscope by direct visual examination of single cells or by examining photographic prints. When an ac field reached the intensity, Ecrit, that generated a maximal membrane potential delta psi max, equal to or greater than the delta psi crit, the membrane was perforated at the two loci facing the electrodes. The dye diffused into the cell, giving rise to two bright, narrow bands, which expanded to the whole cell in 1-3 min. delta psi crit's were measured in three media of different resistivities, rho ext, (52,600, 7,050, and 2,380 omega cm), over the range of 0.1-300 kHz, with the field duration of 200 ms. Regression analysis based on the Schwan Equation showed that in a medium of given resistivity, the delta psi crit was constant over the frequency range studied. When the capacitance of the membrane, Cmembr, was taken to be 0.90 microF cm-2, the resistivity of the cytoplasmic medium, rho int, was determined to be 910-1,100 omega cm. The delta psi crit were 0.33, 0.48, and 0.53 V, respectively, for the three media in decreasing resistivities. The good fit of these data to the curves calculated using the Schwan Equation indicates that the equation may be used to describe the transmembrane potential of a living cell generated by an oscillating electric field.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartoletti D. C., Harrison G. I., Weaver J. C. The number of molecules taken up by electroporated cells: quantitative determination. FEBS Lett. 1989 Oct 9;256(1-2):4–10. doi: 10.1016/0014-5793(89)81707-7. [DOI] [PubMed] [Google Scholar]
- Bernhardt J., Pauly H. On the generation of potential differences across the membranes of ellipsoidal cells in an alternating electrical field. Biophysik. 1973;10(3):89–98. doi: 10.1007/BF01189915. [DOI] [PubMed] [Google Scholar]
- Chang D. C., Reese T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Sukharev S. I., Popov S. V., Pastushenko V. F., Sokirko A. V., Abidor I. G., Chizmadzhev Y. A. The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochim Biophys Acta. 1987 Sep 3;902(3):360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- Coster H. G. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of "punch-through". Biophys J. 1965 Sep;5(5):669–686. doi: 10.1016/S0006-3495(65)86745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. Membrane electroporation--fast molecular exchange by electroosmosis. Biochim Biophys Acta. 1990 Mar;1022(3):381–392. doi: 10.1016/0005-2736(90)90289-z. [DOI] [PubMed] [Google Scholar]
- Ehrenberg B., Farkas D. L., Fluhler E. N., Lojewska Z., Loew L. M. Membrane potential induced by external electric field pulses can be followed with a potentiometric dye. Biophys J. 1987 May;51(5):833–837. doi: 10.1016/S0006-3495(87)83410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D., Loew L. M., Webb W. W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986 Aug;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG C., WHEELDON L., THOMPSON T. E. THE PROPERTIES OF LIPID BILAYER MEMBRANES SEPARATING TWO AQUEOUS PHASES: FORMATION OF A MEMBRANE OF SIMPLE COMPOSITION. J Mol Biol. 1964 Jan;8:148–160. doi: 10.1016/s0022-2836(64)80155-8. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. T. Hemolysis of human erythrocytes by transient electric field. Proc Natl Acad Sci U S A. 1977 May;74(5):1923–1927. doi: 10.1073/pnas.74.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature. 1977 Aug 4;268(5619):438–441. doi: 10.1038/268438a0. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Tsong T. Y. Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim Biophys Acta. 1977 Dec 1;471(2):227–242. doi: 10.1016/0005-2736(77)90252-8. [DOI] [PubMed] [Google Scholar]
- Lo M. M., Tsong T. Y., Conrad M. K., Strittmatter S. M., Hester L. D., Snyder S. H. Monoclonal antibody production by receptor-mediated electrically induced cell fusion. 1984 Aug 30-Sep 5Nature. 310(5980):792–794. doi: 10.1038/310792a0. [DOI] [PubMed] [Google Scholar]
- Mouneimne Y., Tosi P. F., Gazitt Y., Nicolau C. Electro-insertion of xeno-glycophorin into the red blood cell membrane. Biochem Biophys Res Commun. 1989 Feb 28;159(1):34–40. doi: 10.1016/0006-291x(89)92400-5. [DOI] [PubMed] [Google Scholar]
- Rossignol D. P., Decker G. L., Lennarz W. J., Tsong T. Y., Teissie J. Induction of calcium-dependent, localized cortical granule breakdown in sea-urchin eggs by voltage pulsation. Biochim Biophys Acta. 1983 Dec 19;763(4):346–355. doi: 10.1016/0167-4889(83)90096-4. [DOI] [PubMed] [Google Scholar]
- Sale A. J., Hamilton W. A. Effects of high electric fields on micro-organisms. 3. Lysis of erythrocytes and protoplasts. Biochim Biophys Acta. 1968 Aug;163(1):37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. Fusion events and nonfusion contents mixing events induced in erythrocyte ghosts by an electric pulse. Biophys J. 1988 Oct;54(4):619–626. doi: 10.1016/S0006-3495(88)82997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Sowers A. E. Movement of a fluorescent lipid label from a labeled erythrocyte membrane to an unlabeled erythrocyte membrane following electric-field-induced fusion. Biophys J. 1985 Apr;47(4):519–525. doi: 10.1016/S0006-3495(85)83946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissie J., Tsong T. Y. Electric field induced transient pores in phospholipid bilayer vesicles. Biochemistry. 1981 Mar 17;20(6):1548–1554. doi: 10.1021/bi00509a022. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electrical modulation of membrane proteins: enforced conformational oscillations and biological energy and signal transductions. Annu Rev Biophys Biophys Chem. 1990;19:83–106. doi: 10.1146/annurev.bb.19.060190.000503. [DOI] [PubMed] [Google Scholar]
- Weaver J. C., Astumian R. D. The response of living cells to very weak electric fields: the thermal noise limit. Science. 1990 Jan 26;247(4941):459–462. doi: 10.1126/science.2300806. [DOI] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]
- Yeh C. J., Hsi B. L., Faulk W. P. Propidium iodide as a nuclear marker in immunofluorescence. II. Use with cellular identification and viability studies. J Immunol Methods. 1981;43(3):269–275. doi: 10.1016/0022-1759(81)90174-5. [DOI] [PubMed] [Google Scholar]