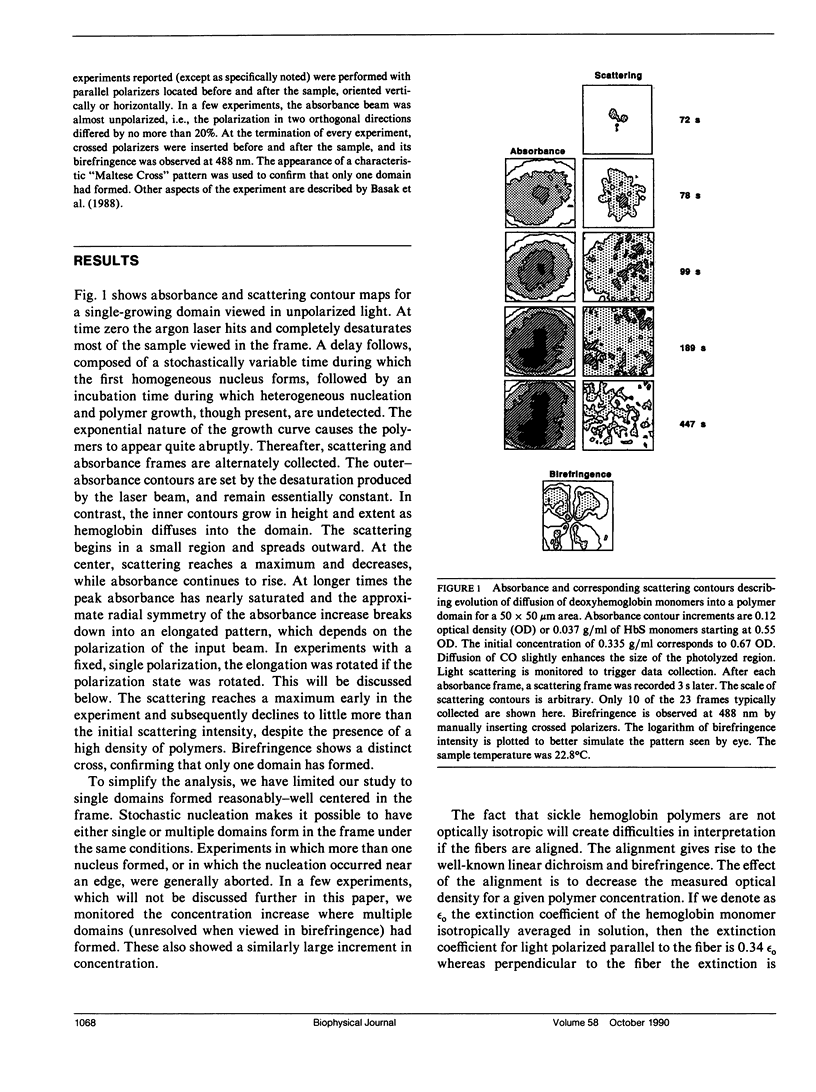
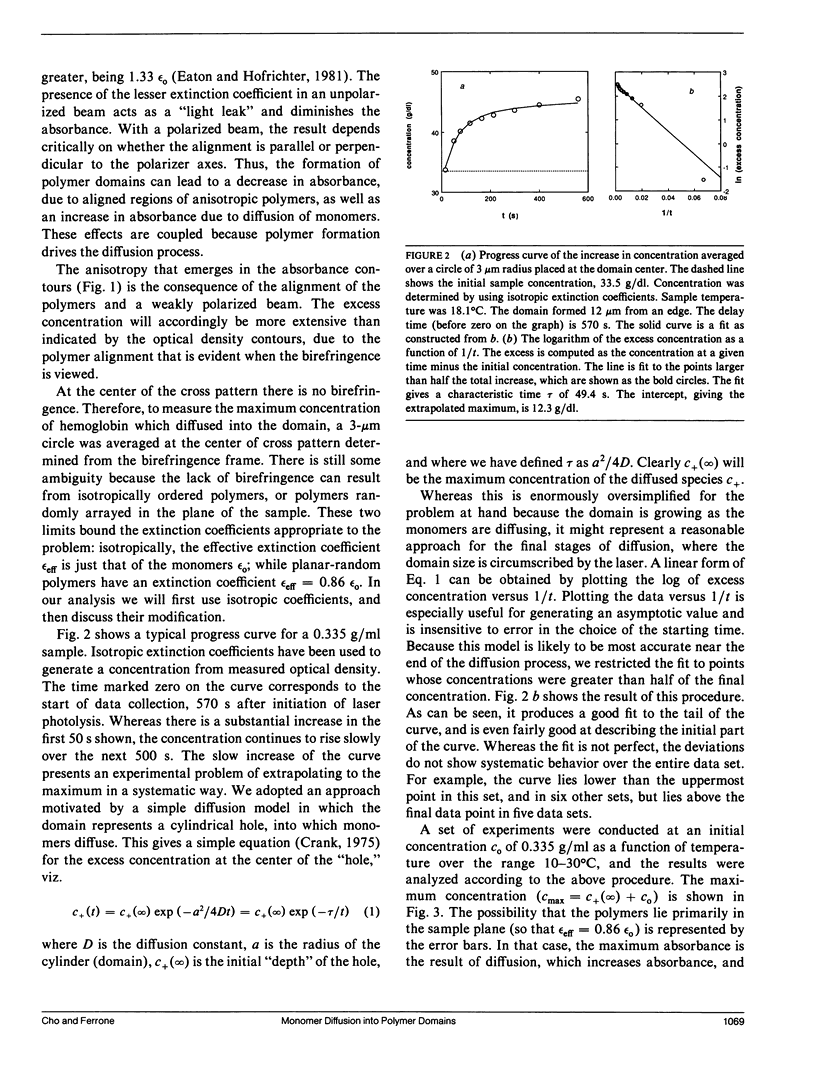
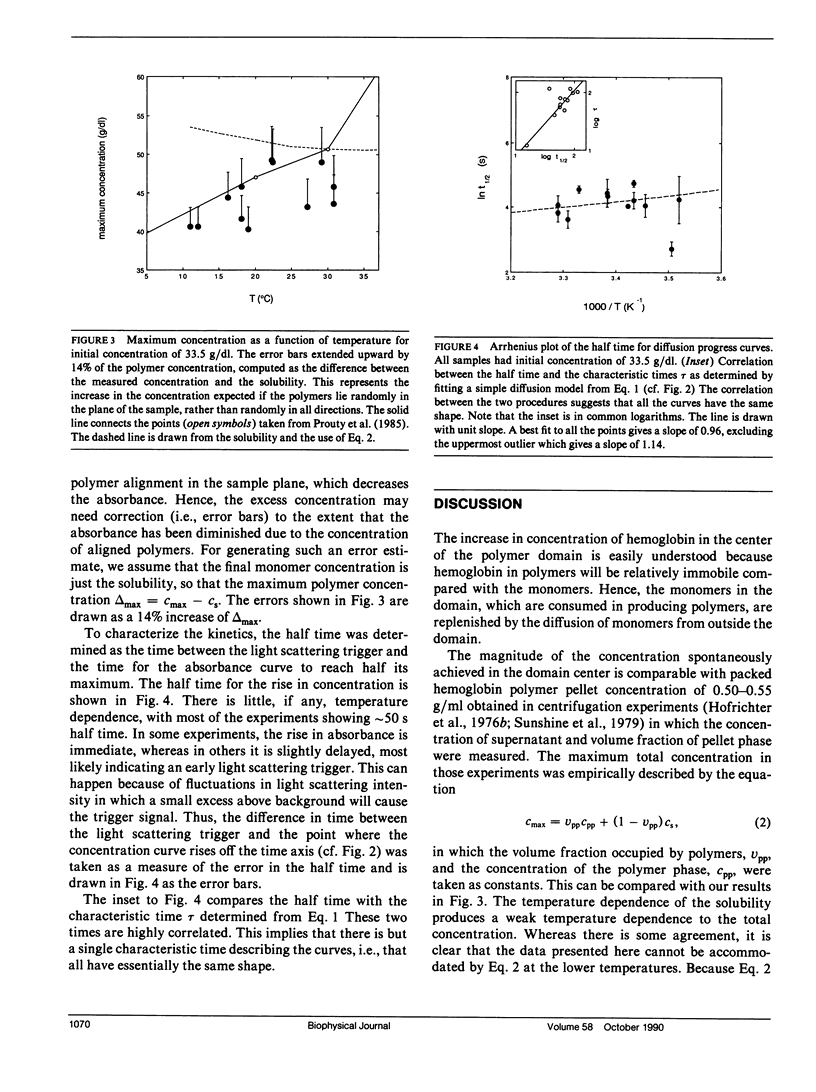
Abstract
The gelation of sickle hemoglobin includes the formation of spherulitic arrays of polymers, known as polymer domains, which are an intrinsic result of the polymer formation mechanism. We have observed the diffusion of monomers into domains as they form, which substantially increases the total concentration of hemoglobin within the domain. The maximum total concentration attained is comparable with the pellet concentration of 0.5-0.55 g/cm3 obtained in sedimentation experiments. The half time for this process is approximately 50 s for domains of 25 microns radius, and is approximately independent of temperature. The shape of the diffusion progress curves as well as the deduced diffusion constants, and their weak temperature dependence are consistent with a simple model of hemoglobin monomer diffusion into the domain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basak S., Ferrone F. A., Wang J. T. Kinetics of domain formation by sickle hemoglobin polymers. Biophys J. 1988 Nov;54(5):829–843. doi: 10.1016/S0006-3495(88)83020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987 Nov;70(5):1245–1266. [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A., Hofrichter J., Eaton W. A. Kinetics of sickle hemoglobin polymerization. I. Studies using temperature-jump and laser photolysis techniques. J Mol Biol. 1985 Jun 25;183(4):591–610. doi: 10.1016/0022-2836(85)90174-3. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A., Hofrichter J., Eaton W. A. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J Mol Biol. 1985 Jun 25;183(4):611–631. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A., Hofrichter J., Sunshine H. R., Eaton W. A. Kinetic studies on photolysis-induced gelation of sickle cell hemoglobin suggest a new mechanism. Biophys J. 1980 Oct;32(1):361–380. doi: 10.1016/S0006-3495(80)84962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone F. A. Kinetic models and the pathophysiology of sickle cell disease. Ann N Y Acad Sci. 1989;565:63–74. doi: 10.1111/j.1749-6632.1989.tb24151.x. [DOI] [PubMed] [Google Scholar]
- Gill S. J., Benedict R. C., Fall L., Spokane R., Wyman J. Oxygen binding to sickle cell hemoglobin. J Mol Biol. 1979 May 15;130(2):175–189. doi: 10.1016/0022-2836(79)90425-x. [DOI] [PubMed] [Google Scholar]
- Hofrichter J. Kinetics of sickle hemoglobin polymerization. III. Nucleation rates determined from stochastic fluctuations in polymerization progress curves. J Mol Biol. 1986 Jun 5;189(3):553–571. doi: 10.1016/0022-2836(86)90324-4. [DOI] [PubMed] [Google Scholar]
- Hofrichter J. Ligand binding and the gelation of sickle cell hemoglobin. J Mol Biol. 1979 Mar 5;128(3):335–369. doi: 10.1016/0022-2836(79)90092-5. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. R., Johnson C. S., Jr Photon correlation spectroscopy of hemoglobin: diffusion of oxy-HbA and oxy-HbS. Biopolymers. 1978 Jun;17(6):1581–1593. doi: 10.1002/bip.1978.360170615. [DOI] [PubMed] [Google Scholar]
- Kam Z., Hofrichter J. Quasi-elastic laser light scattering from solutions and gels of hemoglobin S. Biophys J. 1986 Nov;50(5):1015–1020. doi: 10.1016/S0006-3495(86)83544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty M. S., Schechter A. N., Parsegian V. A. Chemical potential measurements of deoxyhemoglobin S polymerization. Determination of the phase diagram of an assembling protein. J Mol Biol. 1985 Aug 5;184(3):517–528. doi: 10.1016/0022-2836(85)90298-0. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Eaton W. A. Gelation of sickle cell hemoglobin in mixtures with normal adult and fetal hemoglobins. J Mol Biol. 1979 Oct 9;133(4):435–467. doi: 10.1016/0022-2836(79)90402-9. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Ferrone F. A., Eaton W. A. Oxygen binding by sickle cell hemoglobin polymers. J Mol Biol. 1982 Jun 25;158(2):251–273. doi: 10.1016/0022-2836(82)90432-6. [DOI] [PubMed] [Google Scholar]
- White J. G., Heagan B. The fine structure of cell-free sickled hemoglobin. Am J Pathol. 1970 Jan;58(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- Zhou H. X., Ferrone F. A. Theoretical description of the spatial dependence of sickle hemoglobin polymerization. Biophys J. 1990 Sep;58(3):695–703. doi: 10.1016/S0006-3495(90)82412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


