Abstract
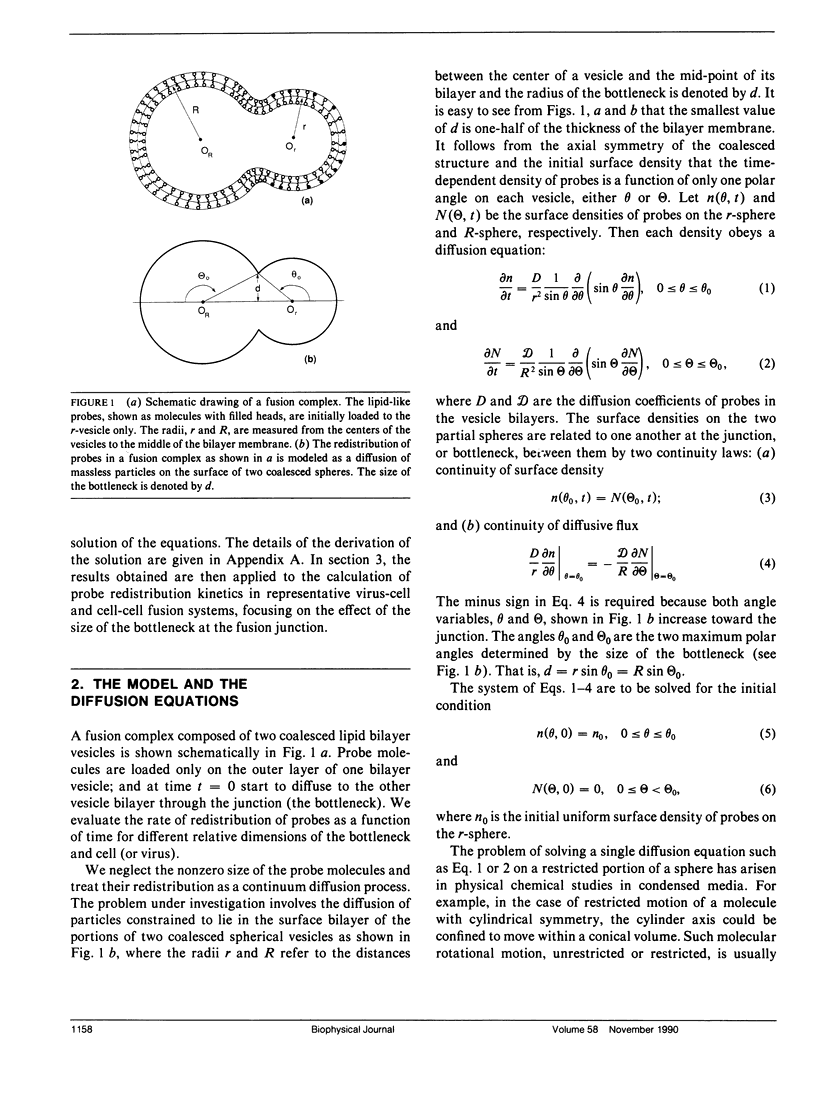
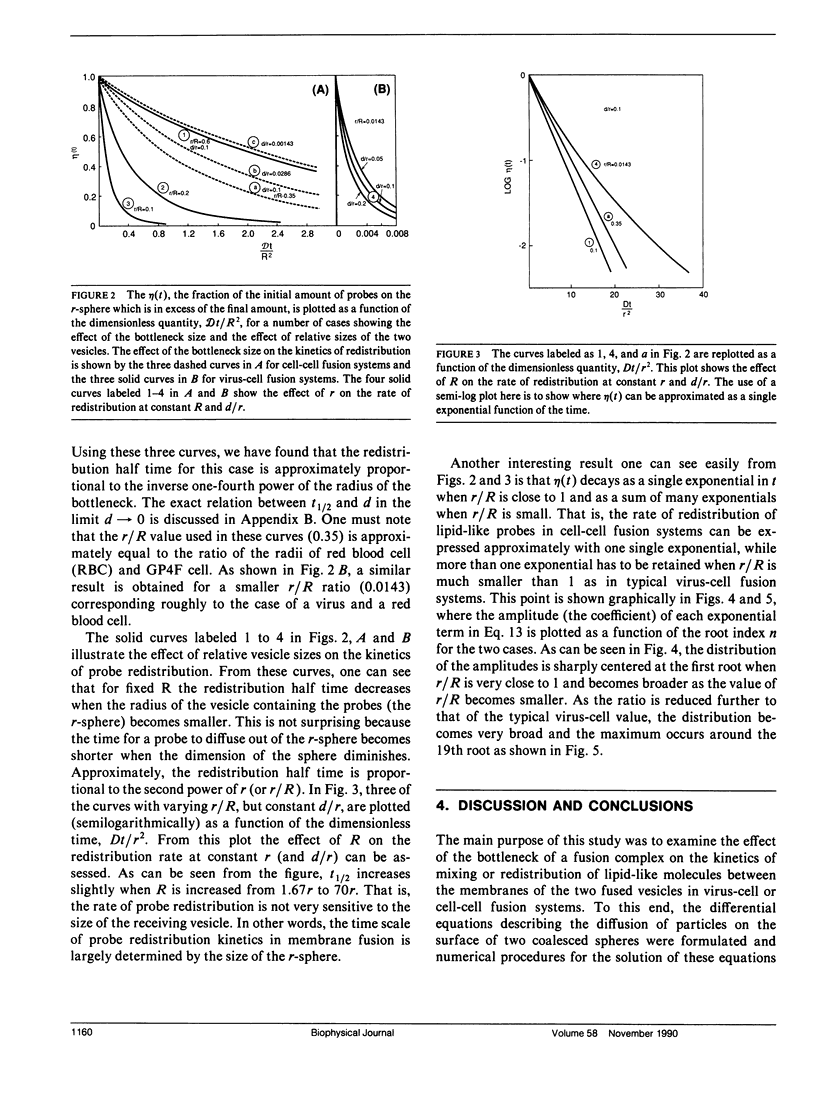
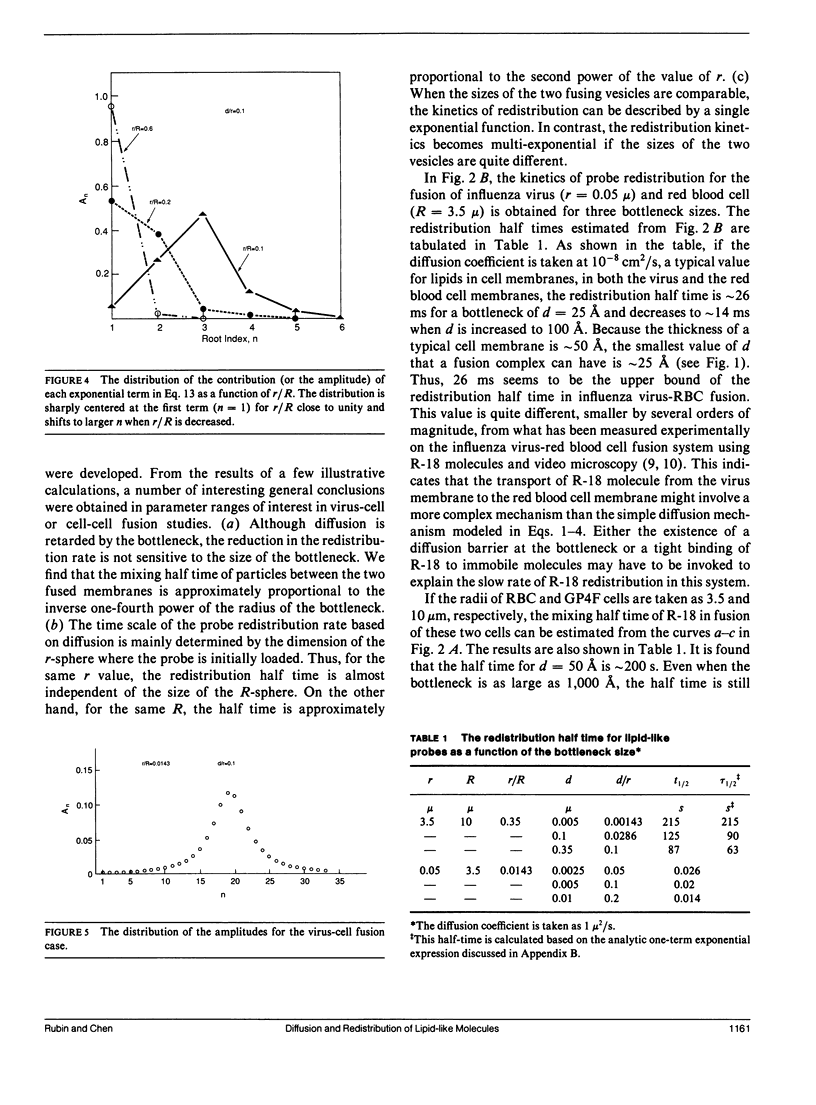
The kinetics of redistribution of lipid-like molecules between the membranes of two fused spherical vesicles is studied by solving the time-dependent diffusion equation of the system. The effects on the probe redistribution rate of pore size at the fusion junction and the relative sizes of the vesicles are examined. It is found that the redistribution rate constant decreases significantly, but not drastically, as the relative size of the pore to that of the vesicles decreases (the bottleneck effect). In general, the time scale of the probe redistribution rate is determined by the size of the vesicles that is loaded with the probe before the activation of the fusion. For a pore size 50 A in diameter and a typical diffusion coefficient of 10(-8) cm2/s for lipids, the mixing half times for typical virus-cell and cell-cell fusion systems are less than 30 ms and above 200 s, respectively. Thus, although the redistribution of lipid-like probes by diffusion is not rate limiting in virus-cell fusion, redistribution by diffusion is close to rate limiting in spike-protein mediated cell-cell fusion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R., Bali-Puri A., Walter A., Covell D., Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J Biol Chem. 1987 Oct 5;262(28):13614–13619. [PubMed] [Google Scholar]
- Chen Y. D., Blumenthal R. On the use of self-quenching fluorophores in the study of membrane fusion kinetics. The effect of slow probe redistribution. Biophys Chem. 1989 Nov;34(3):283–292. doi: 10.1016/0301-4622(89)80065-1. [DOI] [PubMed] [Google Scholar]
- Georgiou G. N., Morrison I. E., Cherry R. J. Digital fluorescence imaging of fusion of influenza virus with erythrocytes. FEBS Lett. 1989 Jul 3;250(2):487–492. doi: 10.1016/0014-5793(89)80782-3. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K. Use of a fluorescence assay to monitor the kinetics of fusion between erythrocyte ghosts, as induced by Sendai virus. Biosci Rep. 1986 Nov;6(11):953–960. doi: 10.1007/BF01114971. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Lowy R. J., Sarkar D. P., Chen Y., Blumenthal R. Observation of single influenza virus-cell fusion and measurement by fluorescence video microscopy. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1850–1854. doi: 10.1073/pnas.87.5.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyter A., Citovsky V., Blumenthal R. The use of fluorescence dequenching measurements to follow viral membrane fusion events. Methods Biochem Anal. 1988;33:129–164. doi: 10.1002/9780470110546.ch4. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Sarkar D. P., White J. M., Blumenthal R. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. Measurement by dequenching of fluorescence. J Biol Chem. 1989 Mar 5;264(7):3972–3978. [PubMed] [Google Scholar]
- Puri A., Winick J., Lowy R. J., Covell D., Eidelman O., Walter A., Blumenthal R. Activation of vesicular stomatitis virus fusion with cells by pretreatment at low pH. J Biol Chem. 1988 Apr 5;263(10):4749–4753. [PubMed] [Google Scholar]
- Sarkar D. P., Morris S. J., Eidelman O., Zimmerberg J., Blumenthal R. Initial stages of influenza hemagglutinin-induced cell fusion monitored simultaneously by two fluorescent events: cytoplasmic continuity and lipid mixing. J Cell Biol. 1989 Jul;109(1):113–122. doi: 10.1083/jcb.109.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]


