Abstract
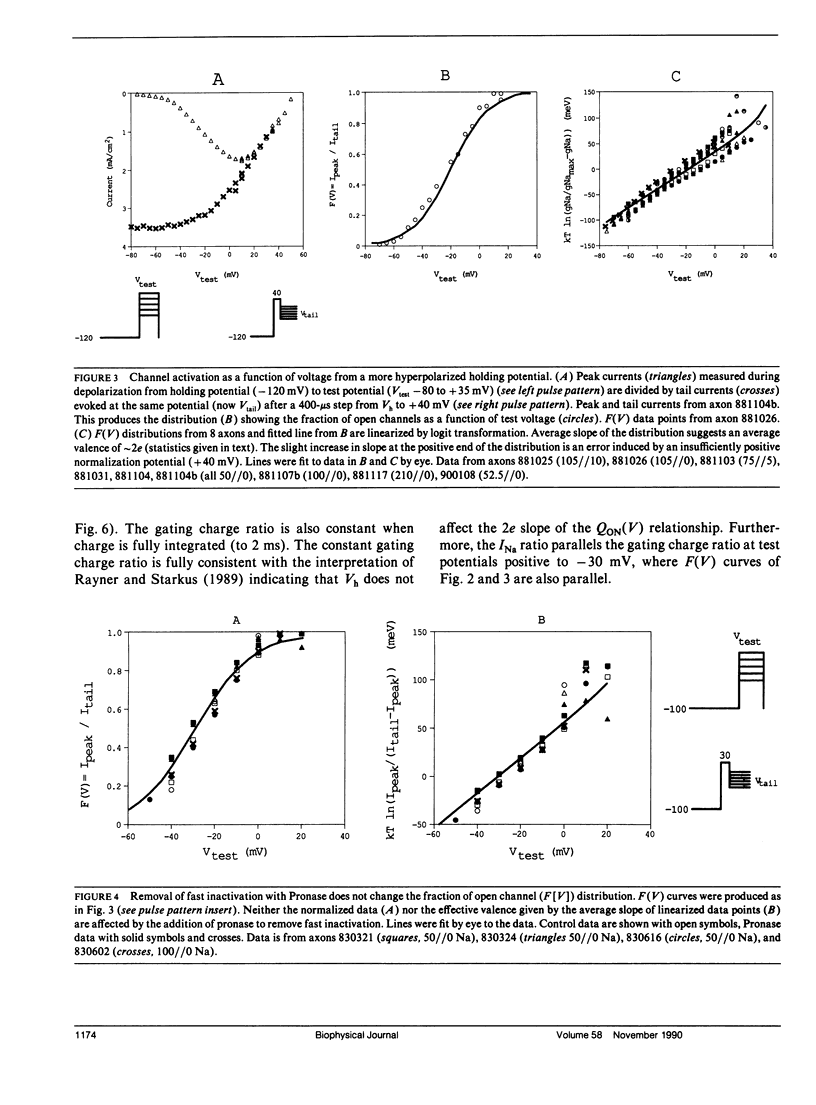
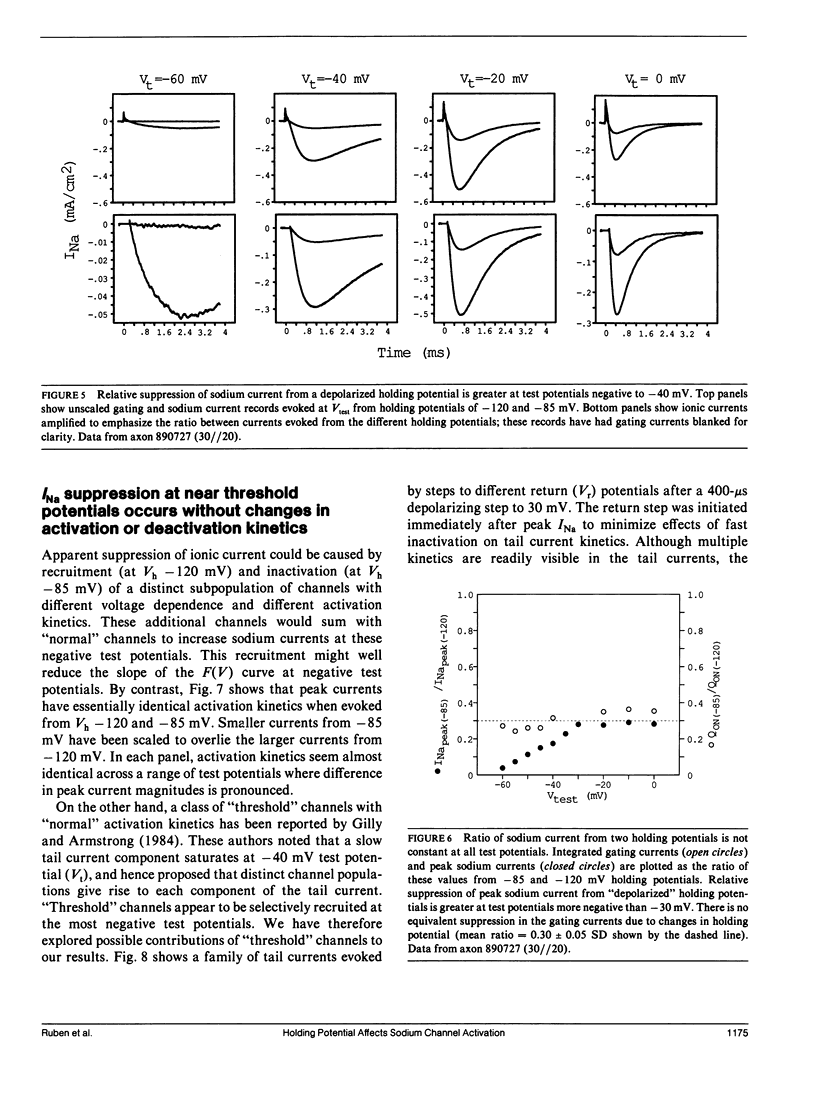
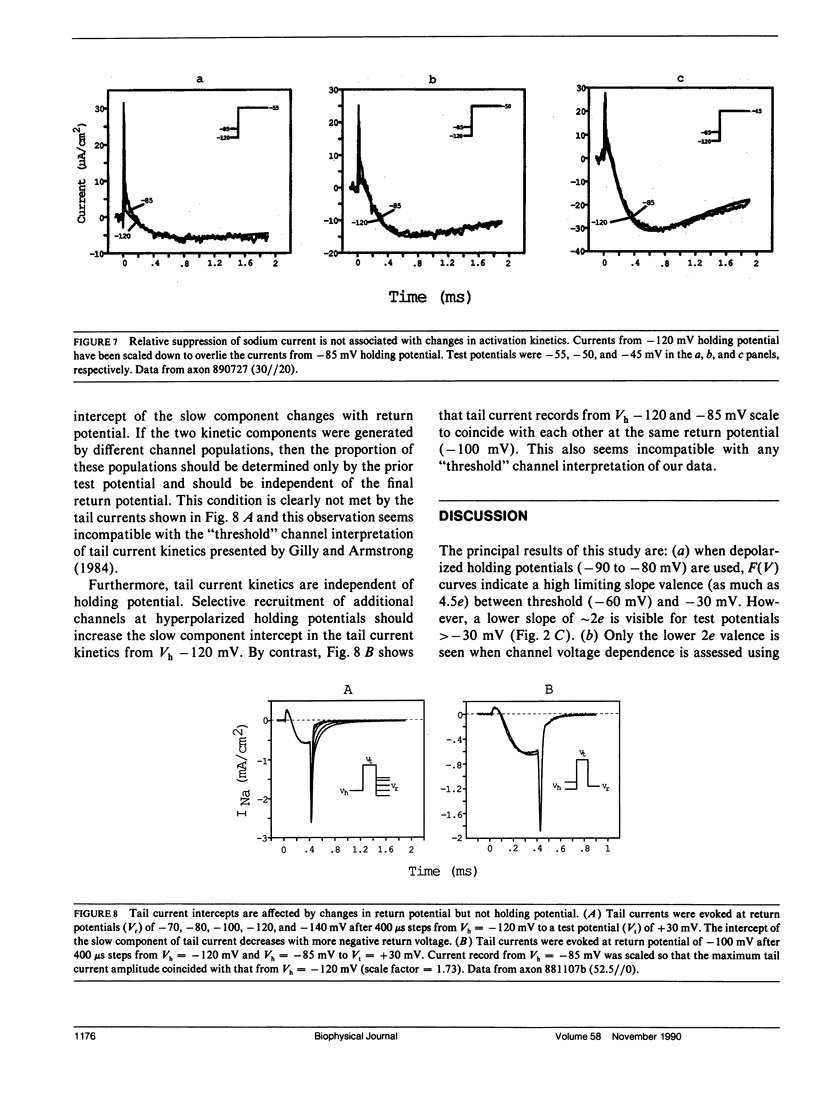
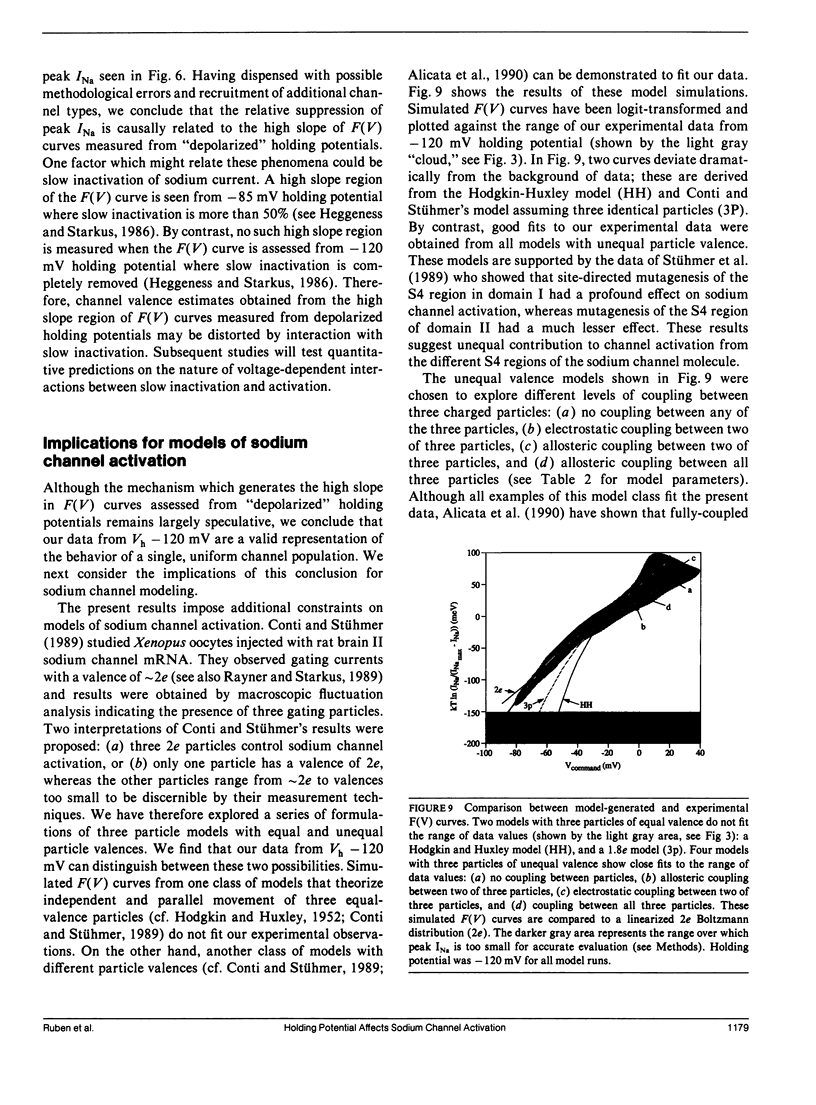
Sodium channel activations, measured as the fraction of channels open to peak conductance for different test potentials (F[V]), shows two statistically different slopes from holding potential more positive than -90 mV. A high valence of 4-6e is indicated a test potentials within 35 mV of the apparent threshold potential (circa -65 mV at -85 mV holding potential). However, for test potentials positive to -30 mV, the F(V) curve shows a 2e valence. The F(V) curve for crayfish axon sodium channels at these "depolarized" holding potentials thus closely resembles classic data obtained from other preparations at holding potentials between -80 and -60 mV. In contrast, at holding potentials more negative than -100 mV, the high slope essentially disappears and the F(V) curve follows a single Boltzmann distribution with a valence of approximately 2e at all potentials. Neither the slope of this simple distribution nor its midpoint (-20 mV) was significantly affected by removal of fast inactivation with pronase. The change in F(V) slope, when holding potential is increased from -85 to -120 mV, does not appear to be caused by the contribution of a second channel type. The simple voltage dependence of sodium current found at Vh -120 mV be used by to discriminate between models of sodium channel activation, and rules out models with three particles of equal valence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alicata D. A., Rayner M. D., Starkus J. G. Sodium channel activation mechanisms. Insights from deuterium oxide substitution. Biophys J. 1990 Apr;57(4):745–758. doi: 10.1016/S0006-3495(90)82595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Properties of the sodium channel gating current. Cold Spring Harb Symp Quant Biol. 1976;40:297–304. doi: 10.1101/sqb.1976.040.01.030. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Taylor R. E., Fernández J. M. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J Gen Physiol. 1982 Jan;79(1):21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Stühmer W. Quantal charge redistributions accompanying the structural transitions of sodium channels. Eur Biophys J. 1989;17(2):53–59. doi: 10.1007/BF00257102. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Threshold channels--a novel type of sodium channel in squid giant axon. 1984 May 31-Jun 6Nature. 309(5967):448–450. doi: 10.1038/309448a0. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol. 1987 Feb;89(2):253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahin R., Campbell D. T. Simple shifts in the voltage dependence of sodium channel gating caused by divalent cations. J Gen Physiol. 1983 Dec;82(6):785–805. doi: 10.1085/jgp.82.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness S. T., Starkus J. G. Saxitoxin and tetrodotoxin. Electrostatic effects on sodium channel gating current in crayfish axons. Biophys J. 1986 Mar;49(3):629–643. doi: 10.1016/S0006-3495(86)83690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D. Properties of the sodium gating current in the squid giant axon. Ann N Y Acad Sci. 1986;479:431–438. doi: 10.1111/j.1749-6632.1986.tb15586.x. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974 Jun;239(2):393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Evidence for a population of sleepy sodium channels in squid axon at low temperature. J Gen Physiol. 1982 May;79(5):739–758. doi: 10.1085/jgp.79.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner M. D., Starkus J. G. The steady-state distribution of gating charge in crayfish giant axons. Biophys J. 1989 Jan;55(1):1–19. doi: 10.1016/S0006-3495(89)82775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Shrager P. Modification of slow sodium inactivation in nerve after internal perfusion with trypsin. Am J Physiol. 1978 Nov;235(5):C238–C244. doi: 10.1152/ajpcell.1978.235.5.C238. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel activation in the squid giant axon. Steady state properties. J Gen Physiol. 1985 Jan;85(1):65–82. doi: 10.1085/jgp.85.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys J. 1984 Jan;45(1):337–344. doi: 10.1016/S0006-3495(84)84159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]



