Abstract
Cytokine mRNA levels were assessed in Burkholderia pseudomallei-susceptible BALB/c mice and B. pseudomallei-resistant C57BL/6 mice following administration of a sublethal dose of less virulent (LV) B. pseudomallei, a candidate immunogen tested for protection against a highly virulent (HV) challenge. Compared on the basis of the bacterial loads, the cytokine patterns induced by HV and LV B. pseudomallei were similar, involving gamma interferon, interleukin-10, and other cytokines. Partial cross-protection between B. pseudomallei strains is shown to be associated with cytokine profiles involving both type 1 and type 2 cytokines.
Melioidosis is a potentially acute fulminating disease of humans and animals caused by the gram-negative intracellular bacterium Burkholderia pseudomallei (36). Infection occurs by subcutaneous inoculation of contaminated soil or surface water and can result in latent infection or a diverse range of clinical presentations (6). As recently reported by Currie et al. (8), recrudescence of disease may occur years after initial exposure, which suggests that the immune responses induced may not be effective for clearance of the organism. Differences in host and pathogen virulence factors are known to be important in determining disease severity (33). Gamma interferon (IFN-γ) plays a critical role in innate host resistance during primary infection (26), but mechanisms of adaptive immunity have not been widely studied. BALB/c mice are susceptible to infection with highly virulent (HV) B. pseudomallei, whereas C57BL/6 mice are relatively resistant (17). Cytokine responses following an HV B. pseudomallei challenge (34) are unlike those seen in other models of intracellular infection, and resistance and susceptibility do not correlate with divergent cytokine profiles commonly associated with development of distinct T helper cell subsets. Instead, early cytokine profiles are similar in BALB/c and C57BL/6 mice, with the only demonstrated difference being in the magnitude of the cytokine response (34). Responses involving a similar range of cytokines have been reported in patients (4, 11, 31).
Characterization of less virulent (LV) strains of B. pseudomallei (12, 13, 35) allows us to determine their potential as candidate immunogens for induction of adaptive immunity against HV B. pseudomallei. In models of infection with other intracellular pathogens (3, 5, 22, 27, 28, 29), cytokine responses are dependent upon the level of virulence of the challenge strain. The present study investigated the influence of B. pseudomallei virulence on disease progression and cytokine responses in melioidosis. Cytokine profiling of the spleen and liver was carried out by reverse transcription-PCR, and bacterial growth in the blood, liver, and spleen was determined. Immunization experiments were performed by using LV B. pseudomallei as a candidate immunogen for protection against an HV challenge to analyze the relationship between cytokine responses and immunity to B. pseudomallei.
The B. pseudomallei strains used were NCTC 13178 (HV) and NCTC 13179 (LV). The virulence of the strains has been described previously (33). B. pseudomallei strain ATCC 23343 was used as a reference strain in immunization experiments. For cytokine studies, C57BL/6 and BALB/c mice (8 to 16 weeks old) were administered 25 CFU of either HV or LV B. pseudomallei in 200 μl of phosphate-buffered saline (PBS) intravenously (i.v.). At various times following inoculation, five mice per group were euthanized with CO2 and the bacterial load in the blood was determined (17). The liver and spleen were excised, and one half was used to determine the bacterial load (17) while the other half was stored at −80°C until RNA extraction. A second model of primary infection was established with a larger inoculum (6 × 104 CFU) of LV B. pseudomallei. This model allowed a comparison of HV and LV B. pseudomallei at equivalent bacterial loads. RNA was extracted from the liver with TRIZOL reagent (Life Technologies) (7, 34) and from the spleen with SV total RNA isolation spin columns (Promega). DNase treatment, RNA quantification, reverse transcription, and PCR with cytokine-specific primers were performed as previously described (34). For lipopolysaccharide-induced CXC chemokine (LIX), primer sequences were designed with OLIGO v. 5 software (24) and PCR parameters were optimized with a plasmid containing LIX cDNA, which was transformed into competent Escherichia coli strain JM109 as previously described (14, 25). The primers 5′ TCC AGC TCG CCA TTC A 3′ (sense) and 5′ TCC GCT TAG CTT TCT TTT TG 3′ (antisense) were designed to amplify a 319-bp LIX product. LIX PCR products were gel purified with a QIAQUICK gel extraction kit (Qiagen) and sequenced with Big Dye Terminator kit (Perkin-Elmer) on an ABI 310 sequencer (Perkin-Elmer). Sequences were checked for homology with previously described gene sequences for LIX (30). The PCR was repeated twice for three separately prepared cDNA samples.
For immunization experiments, groups of 10 mice were administered either PBS (nonimmunized) or 0.1 50% lethal dose (LD50) of LV B. pseudomallei NCTC 13179 or reference strain ATCC 23343 (33). Two weeks later, mice were challenged with one of three different doses of HV B. pseudomallei NCTC 13178 (10 LD50s, 1 LD50, or 0.1 LD50). Additional control groups received PBS (negative controls). Another series of five mice were immunized and challenged as already described and subsequently used to determine the bacterial load in the spleen at 72 h after a secondary challenge. Statistical analysis of bacterial load data was performed with a two-tailed Student t test for independent samples. Data are expressed as the mean ± the standard error of the mean. Mortalities in immunized versus nonimmunized groups were analyzed by the Mann-Whitney U test. P values of <0.01 were considered significant.
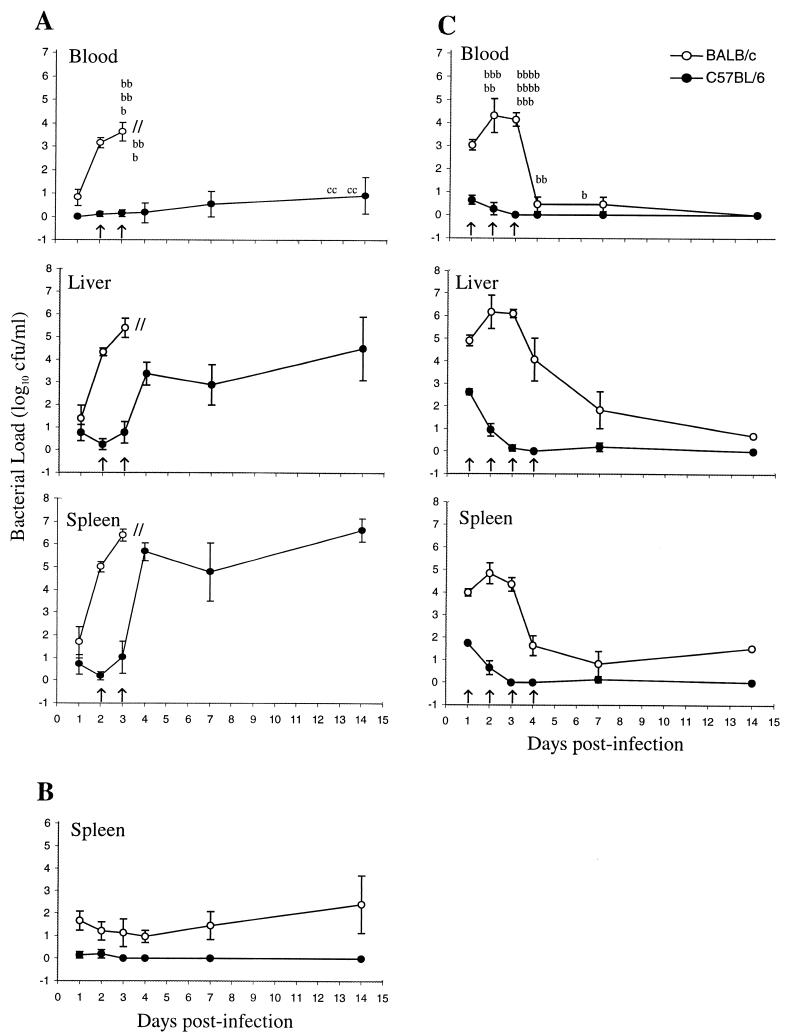
HV B. pseudomallei replicated exponentially in the blood, livers, and spleens of BALB/c mice until host death at 72 h (Fig. 1A). LV B. pseudomallei (25 CFU) was effectively contained in all of the organs (Fig. 1B; data not shown). In mice administered 6 × 104 CFU, exponential growth of LV B. pseudomallei occurred in BALB/c mice until 72 h, in contrast to C57BL/6 mice, which reduced the challenge inoculum (Fig. 1C). BALB/c mice that survived the early phase of infection rapidly reduced the bacterial load (Fig. 1C). The reduction in the bacterial load of BALB/c mice that survived the high-level challenge with LV B. pseudomallei suggests the potential for the development of an appropriate immune response in these innately susceptible mice. Due to progressive deaths of BALB/c mice, the day 14 data (Fig. 1C) represent one mouse.
FIG. 1.
Growth of HV B. pseudomallei NCTC 13178 (A) and LV B. pseudomallei NCTC 13179 (25-CFU [B] and 6 × 104-CFU [C] challenges) in BALB/c and C57BL/6 mice. Results are shown as the mean bacterial load (log10 CFU per milliliter) of five mice ± the standard error of the mean. Significant differences (P < 0.01) are indicated by arrows. b, BALB/c mouse death; c, C57BL/6 mouse death.
Cytokine responses in the liver were most pronounced in mice challenged with either HV B. pseudomallei or the larger dose of LV B. pseudomallei (Fig. 2). Responses in the liver involved IFN-γ, tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-10 (Fig. 2) and correlated with the bacterial load (Fig. 1). In mice administered 25 CFU of LV B. pseudomallei, absence of a detectable cytokine responses in the liver was associated with a light bacterial load (data not shown). This indicates a requirement for a substantial B. pseudomallei load in the liver before induction of a local cytokine responses. LIX mRNA was detected in the livers of BALB/c, but not C57BL/6, mice during infection (Fig. 2). No mRNA for IL-2 or IL-4 was detected in the livers of mice following a challenge with B. pseudomallei (data not shown).
FIG. 2.
Production of cytokine mRNA in livers of BALB/c and C57BL/6 mice infected i.v. with either 25 CFU of HV B. pseudomallei NCTC 13178 (a) or 6 × 104 CFU of LV B. pseudomallei NCTC 13179 (b). The values on the left are the molecular sizes of the markers in lanes M in kilodaltons.
In the spleen, cytokine responses involved IFN-γ, TNF-α, IL-1β, IL-4, and IL-12 (p40 results are shown, but similar results were obtained with p35) and also correlated with the bacteria load (Fig. 1). LIX mRNA was detected in the spleens of BALB/c, but not C57BL/6, mice infected with HV B. pseudomallei (Fig. 3). The TNF-α and IL-12 responses in the spleens of C57BL/6 mice infected with 6 × 104 CFU of LV B. pseudomallei (Fig. 3) did not correlate with the bacterial load (Fig. 1). Instead, mRNAs for TNF-α, IL-12, and IL-4 in these mice were not detected until 48 to 96 h (Fig. 3), when the bacterial load was <101 CFU/ml (Fig. 1). IL-4 also demonstrated delayed induction kinetics in the spleens of BALB/c mice challenged with 6 × 104 CFU of LV B. pseudomallei (Fig. 3). IL-2 mRNA was not detected in the spleens of mice of either strain (data not shown). Twenty-five CFU of LV B. pseudomallei induced cytokine responses in the spleen that were similar but less pronounced than those induced by HV B. pseudomallei. The association between the cytokine mRNAs and the bacterial loads in the spleens of these mice did not follow the same trend as that observed with HV B. pseudomallei. For example, responses in BALB/c mice at 48 to 96 h coincided with bacterial loads of ≤102 CFU/ml. Equivalent bacterial loads in the liver at 48 to 96 h were not associated with cytokine responses (data not shown). In contrast to BALB/c mice, C57BL/6 mice infected with 25 CFU of LV B. pseudomallei demonstrated no increase in splenic IL-10 mRNA (Fig. 3). Preferential expression of IL-10 in BALB/c mice suggests a possible role for this cytokine in innate susceptibility to B. pseudomallei infection.
FIG. 3.
Production of cytokine mRNA in spleens of BALB/c and C57BL/6 mice infected i.v. with either 25 CFU of HV B. pseudomallei NCTC 13178 (a), 25 CFU of LV B. pseudomallei NCTC 13179 (b), or 6 × 104 CFU of B. pseudomallei NCTC 13179 (c). The values on the left are the molecular sizes of the markers in lanes M in kilodaltons.
Partial immunoprotection was demonstrated in mice immunized with either NCTC 13179 or reference strain ATCC 23343 (Fig. 4A). Immunized mice did not develop symptoms of disease prior to a secondary challenge, despite the recovery of viable B. pseudomallei from the majority of the animals at day 14. Significantly better protection was obtained with NCTC 13179 (P = 0.0001) than with ATCC 23343 (P = 0.143). Bacterial load data from immunized mice (Fig. 4B) confirmed partial, rather than sterilizing, immunity. Bacterial loads at 72 h after a secondary challenge of mice immunized with either NCTC 13179 or ATCC 23343 were not significantly different from those of nonvaccinated controls (Fig. 4B). These studies, however, cannot discriminate which strain is the one cultured after a secondary challenge, and hence, it is possible that an HV challenge could change host immunity so that increased replication of LV B. pseudomallei may occur.
FIG. 4.
LV B. pseudomallei NCTC 13179 and reference strain ATCC 23343 confer partial protection against HV B. pseudomallei NCTC 13178 (10-LD50 challenge data are shown). Significant differences in mortality (A) between mice immunized with LV B. pseudomallei NCTC 13179 and nonimmunized controls are indicated (∗, P = 0.0001). The bacterial loads in the spleens of mice immunized with either LV B. pseudomallei NCTC 13179 or ATCC 23343 and rechallenged with HV B. pseudomallei demonstrate partial protection rather than sterilizing immunity (B). Although the bacterial loads of immunized C57BL/6 mice were, on average, lighter than those of nonimmunized controls, these differences were not statistically significant (∗, P = 0.084 for NCTC 13179 and P = 0.05 for ATCC 23343).
This study demonstrates an overall similarity between the cytokine profiles induced by B. pseudomallei strains with contrasting levels of virulence when compared on the basis of bacterial loads. These observations are in contrast to reports on other intracellular pathogens (2, 5, 15, 21, 27, 29). This study also demonstrates an association between these responses and partial cross-protection of mice immunized with LV B. pseudomallei and challenged with HV B. pseudomallei. Early IFN-γ and IL-12 responses in the spleens of immunized mice, combined with low-level persistence of viable LV B. pseudomallei, were considered potentially beneficial for the stimulation of a more effective host response for adaptive immunity against HV B. pseudomallei. Although immunized mice demonstrated significantly better survival after a subsequent challenge with HV B. pseudomallei than did nonimmunized animals, the protection was not sterilizing, as evidenced by the recovery of high numbers of bacteria from the spleen. Also, even though the cytokine patterns induced by HV and LV B. pseudomallei are similar, the magnitude of the response to LV B. pseudomallei in the spleen does not simply reflect the bacterial load within the tissue, as predicted on the basis of a model of HV B. pseudomallei infection (34). Although the lack of IL-2 mRNA in the spleens of mice during B. pseudomallei infection cannot be explained, these observations are consistent with recent reports on other intracellular pathogens (10, 18, 20).
High levels of IL-8 have been demonstrated in patients presenting with acute melioidosis (11). A related chemokine in mice is LIX (23, 30, 37). Detection of LIX in BALB/c, but not C57BL/6, mice suggests that LIX may play a role in innate susceptibility to B. pseudomallei infection. To our knowledge, there have been only two previous reports of LIX responses in mice following bacterial infection (19, 32). Lauw et al. (16) demonstrated a limited number of neutrophil-chemoattractant chemokines other than IL-8 in patients infected with B. pseudomallei. Hence, neutrophil-chemoattractant chemokines other than LIX (e.g., ENA-78) may play an important role in the immune response to B. pseudomallei infection. Barnes et al. (1) recently demonstrated diverse chemokine responses in mice infected with B. pseudomallei. The ability of B. pseudomallei to survive and replicate within neutrophils makes the potential role of neutrophil-chemoattractant chemokines in melioidosis particularly interesting (9, 36).
Acknowledgments
This research was supported by The Lions Medical Research Foundation of Australia and program and merit research grants from James Cook University.
We thank Pitak Santanirand and Jeffrey B. Smith for critical review of the manuscript. The LIX cDNA-containing plasmid used in this study was a generous gift from Jeffrey B. Smith and Harvey Herschman of the School of Medicine, University of California, Los Angeles. The competent E. coli JM109 cells used for plasmid transfection were provided by Brendan Cullinane of James Cook University. We thank Leigh Owens for advice on statistical analysis and Scott Blyth for monitoring the experimental animals. We are also grateful to Kellie Powell for excellent technical assistance.
Editor: J. D. Clements
REFERENCES
- 1.Barnes, J. L., G. C. Ulett, N. Ketheesan, T. Clair, P. M. Summers, and R. G. Hirst. 2001. Induction of multiple chemokine and colony-stimulating factor genes in experimental Burkholderia pseudomallei infection. Immunol. Cell Biol. 79:490-501. [DOI] [PubMed] [Google Scholar]
- 2.Blackstock, R., K. L. Buchanan, A. M. Adesina, and J. W. Murphy. 1999. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect. Immun. 67:3601-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, A. E., D. A. B. Dance, Y. Suputtamongkol, W. Chaowagul, S. Kongchareon, H. K. Webster, and N. J. White. 1991. Immune cell activation in melioidosis: increased serum levels of interferon-γ and soluble interleukin-2 receptors without change in soluble CD8 protein. J. Infect. Dis. 163:1145-1148. [DOI] [PubMed] [Google Scholar]
- 5.Caron, E., T. Peyrard, S. Kohler, S. Cabane, P. Liautard, and J. Dornand. 1994. Live Brucella spp. fail to induce tumor necrosis factor excretion upon infection of U937-derived phagocytes. Infect. Immun. 62:5267-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaowagul, W., N. J. White, D. A. B. Dance, Y. Wattanagoon, P. Naigowit, T. M. E. Davis, S. Looareesuwan, and N. Pitakwatchara. 1989. Melioidosis: a major cause of community-acquired septicaemia in northeastern Thailand. J. Infect. Dis. 159:890-898. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 8.Currie, B. J., D. A. Fisher, N. M. Anstey, and S. P. Jacups. 2000. Melioidosis: acute and chronic disease, relapse and re-activation. Trans. R. Soc. Trop. Med. Hyg. 94:301-304. [DOI] [PubMed] [Google Scholar]
- 9.Egan, A. M., and D. L. Gordon. 1996. Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect. Immun. 64:4952-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers, S., M. E. A. Mielke, T. Blankenstein, and H. Hahn. 1992. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. J. Immunol. 149:3016-3022. [PubMed] [Google Scholar]
- 11.Friedland, J. S., Y. Suputtamongkol, D. G. Remick, W. Chaowagul, R. M. Strieter, S. L. Kunkel, N. J. White, and G. E. Griffin. 1992. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect. Immun. 60:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier, Y. P., F. M. Thibault, J. C. Paucod, and D. R. Vidal. 2000. Protease production by Burkholderia pseudomallei and virulence in mice. Acta Trop. 74:215-220. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe, I., B. Brenneke, M. Rohde, A. Kreft, S. Häubler, A. Reganzerowski, and I. Steinmetz. 1999. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 67:2891-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 15.Kasuga-Aoki, H., S. Takai, Y. Sasaki, S. Tsubaki, H. Madarame, and A. Nakane. 1999. Tumor necrosis factor and interferon-gamma are required in host resistance against virulent Rhodococcus equi infection in mice: cytokine production depends on the virulence levels of R. equi. Immunology 96:122-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauw, F. N., A. J. H. Simpson, J. N. Prins, S. J. H. van Deventer, W. Chaowagul, N. J. White, and T. van der Poll. 2000. The CXC chemokines gamma interferon (IFN-γ)-inducible protein 10 and monokine induced by IFN-γ are released during severe melioidosis. Infect. Immun. 68:3888-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leakey, A. K., G. C. Ulett, and R. G. Hirst. 1998. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb. Pathog. 24:269-275. [DOI] [PubMed] [Google Scholar]
- 18.Nabors, G. S., and R. L. Tarleton. 1991. Differential control of IFN-γ and IL-2 production during Trypanosoma cruzi infection. J. Immunol. 146:3591-3598. [PubMed] [Google Scholar]
- 19.Neumann, B., N. Zantl, A. Veihelmann, K. Emmanuilidis, K. Pfeffer, C. D. Heidecke, and B. Holzmann. 1999. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int. Immunol. 11:217-227. [DOI] [PubMed] [Google Scholar]
- 20.Pie, S., P. Truffa-Bachi, M. Pla, and C. Nauciel. 1997. Th1 response in Salmonella typhimurium-infected mice with a high or low rate of bacterial clearance. Infect. Immun. 65:4509-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poston, R. M., and R. J. Kurlander. 1992. Cytokine expression in vivo during murine listeriosis: infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J. Immunol. 149:3040-3044. [PubMed] [Google Scholar]
- 22.Revelli, S., L. Gomez, J. Wietzerbin, O. Bottasso, and M. A. Basombrio. 1999. Levels of tumor necrosis factor alpha, gamma interferon, and interleukins 4, 6, and 10 as determined in mice infected with virulent or attenuated strains of Trypanosoma cruzi. Parasitol. Res. 85:147-150. [DOI] [PubMed] [Google Scholar]
- 23.Rovai, L. E., H. R. Herschman, and J. B. Smith. 1998. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J. Leukoc. Biol. 64:494-502. [DOI] [PubMed] [Google Scholar]
- 24.Rychlik, W., and R. E. Rhoads. 1989. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 17:8543-8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 75-84. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Santanirand, P., V. S. Harley, D. A. B. Dance, B. S. Drasar, and G. J. Bancroft. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarmento, A. M., and R. Appelberg. 1995. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect. Immun. 63:3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarmento, A., and R. Appelberg. 1996. Involvement of reactive oxygen intermediates in tumor necrosis factor alpha-dependent bacteriostasis of Mycobacterium avium. Infect. Immun. 64:3224-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver, R. F., Q. Li, and J. J. Ellner. 1998. Expression of virulence of Mycobacterium tuberculosis within human monocytes: virulence correlates with intracellular growth and induction of tumor necrosis factor alpha but not with evasion of lymphocyte-dependent monocyte effector functions. Infect. Immun. 66:1190-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, J. B., and H. R. Herschman. 1995. Glucocorticoid-attenuated response genes encode intercellular mediators, including a new C-X-C chemokine. J. Biol. Chem. 270:16756-16765. [DOI] [PubMed] [Google Scholar]
- 31.Suputtamongkol, Y., D. Kwiatkowski, D. A. B. Dance, W. Chaowagul, and N. J. White. 1992. Tumor necrosis factor in septicemic melioidosis. J. Infect. Dis. 165:561-564. [DOI] [PubMed] [Google Scholar]
- 32.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68:4289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulett, G. C., B. J. Currie, T. W. Clair, M. Mayo, N. Ketheesan, J. LaBrooy, D. Gal, R. Norton, C. Ashhurst-Smith, J. Barnes, J. Warner, and R. G. Hirst. 2001. Burkholderia pseudomallei virulence: definition, stability and association with clonality. Microbes Infect. 3:621-631. [DOI] [PubMed] [Google Scholar]
- 34.Ulett, G. C., N. Ketheesan, and R. G. Hirst. 2000. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun. 68:2034-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulett, G. C., N. Ketheesan, T. Clair, B. Roberts, J. LaBrooy, R. Norton, C. Ashhurst-Smith, J. Warner, and R. G. Hirst. 2000. Cellular and cytokine responses in experimental melioidosis: virulence influences the induction of TNF-α, IL-1β and IL-6 during the innate phase of immunity, p. 137-144. In S. Sirisinha, S. C. Chaiyaroj, and P. Tapchaisri (ed.), Proceedings of the Second Congress of the Federation of Immunological Societies of Asia-Oceania. Monduzzi Editore S.p.A., Medimond Inc., Milan, Italy.
- 36.Woods, D. E., D. DeShazer, R. A. Moore, P. J. Brett, M. N. Burtnick, S. L. Reckseidler, and M. D. Senkiw. 1999. Current studies on the pathogenesis of melioidosis. Microbes Infect. 1:157-162. [DOI] [PubMed] [Google Scholar]
- 37.Wuyts, A., A. Haelens, P. Proost, J. P. Lenaerts, R. Conings, G. Opdenakker, and J. Van Damme. 1996. Identification of mouse granulocyte chemotactic protein-2 from fibroblasts and epithelial cells: functional comparison with natural KC and macrophage inflammatory protein-2. J. Immunol. 157:1736-1743. [PubMed] [Google Scholar]