Abstract
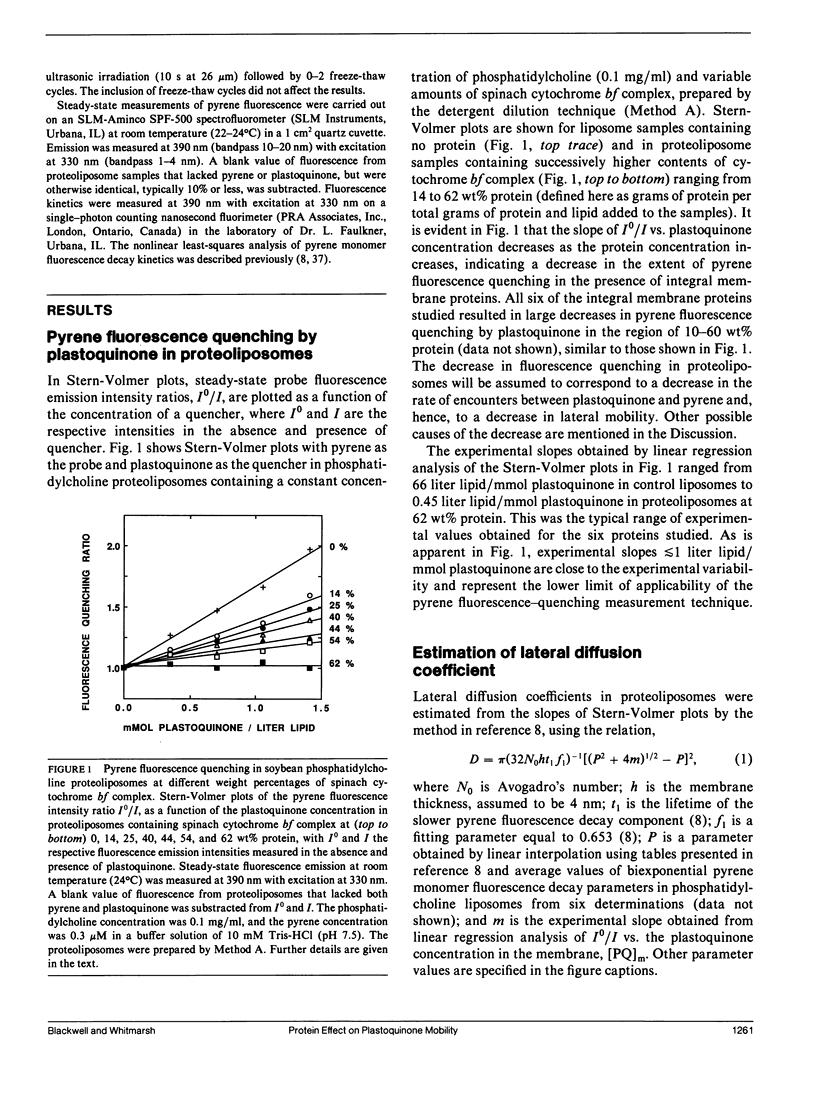
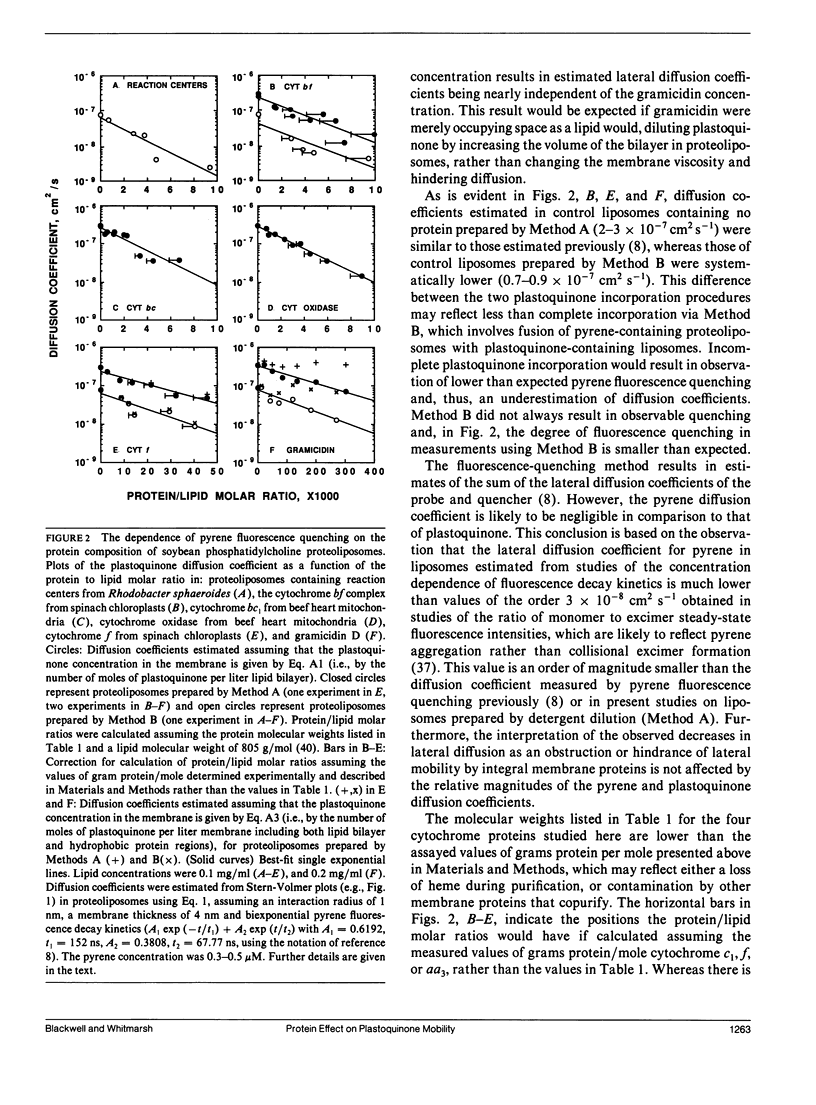
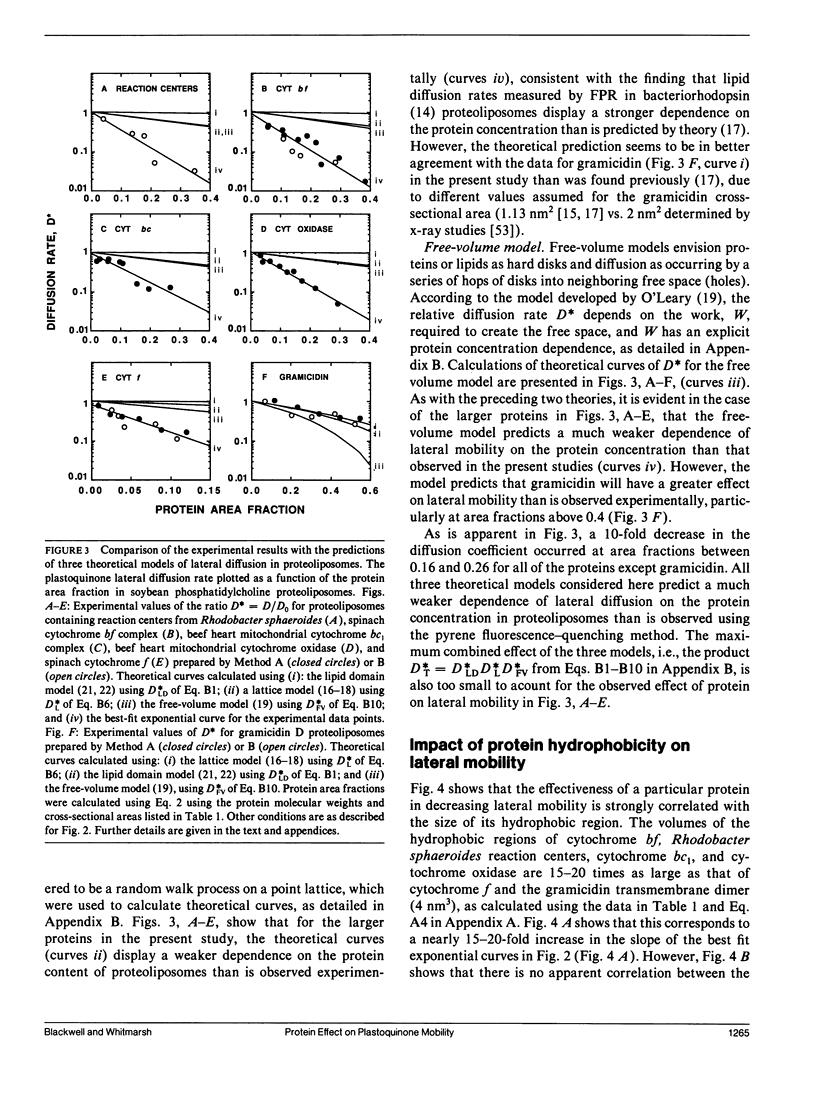
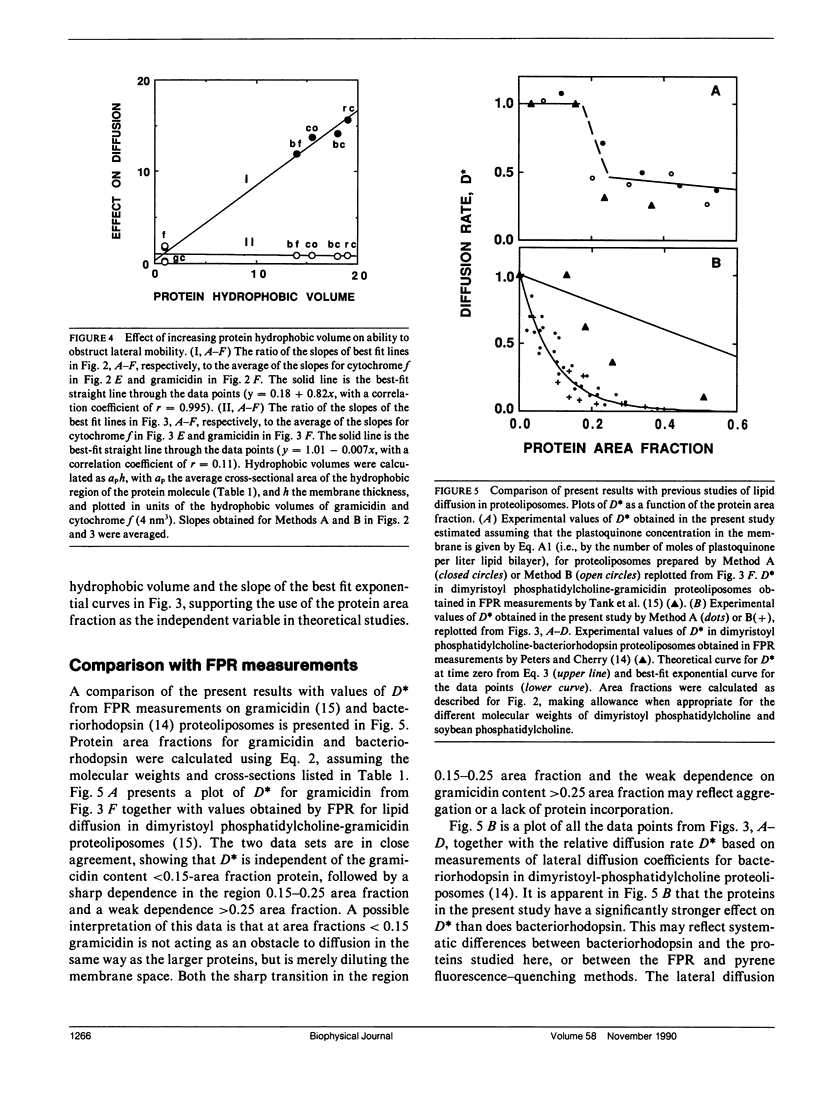
Pyrene fluorescence quenching by plastoquinone was used to estimate the rate of plastoquinone lateral diffusion in soybean phosphatidylcholine proteoliposomes containing the following integral membrane proteins: gramicidin D, spinach cytochrome bf complex, spinach cytochrome f, reaction centers from Rhodobacter sphaeroides, beef heart mitochondrial cytochrome bc1, and beef heart mitochondrial cytochrome oxidase. The measured plastoquinone lateral diffusion coefficient varied between 1 and 3 · 10-7 cm2 s-1 in control liposomes that lacked protein. When proteins were added, these values decreased: a 10-fold decrease was observed when 16-26% of the membrane surface area was occupied by protein for all the proteins but gramicidin. The larger protein complexes (cytochrome bf, Rhodobacter sphaeroides reaction centers, cytochrome bc1, and cytochrome oxidase), whose hydrophobic volumes were 15-20 times as large as that of cytochrome f and the gramicidin transmembrane dimer, were 15-20 times as effective in decreasing the lateral-diffusion coefficient over the range of concentrations studied. These proteins had a much stronger effect than that observed for bacteriorhodopsin in fluorescence photobleaching recovery measurements. The effect of high-protein concentrations in gramicidin proteoliposomes was in close agreement with fluorescence photobleaching measurements. The results are compared with the predictions of several theoretical models of lateral mobility as a function of integral membrane concentration.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Owicki J. C. Self diffusion of interacting membrane proteins. Biophys J. 1989 May;55(5):817–833. doi: 10.1016/S0006-3495(89)82882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Lateral motion of membrane proteins and biological function. J Membr Biol. 1983;75(1):1–10. doi: 10.1007/BF01870794. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Deamer D. W., Cornwell D. G. Surface area of human erythrocyte lipids: reinvestigation of experiments on plasma membrane. Science. 1966 Aug 26;153(3739):1010–1012. doi: 10.1126/science.153.3739.1010. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Blackwell M. F., Gounaris K., Barber J. Evidence that pyrene excimer formation in membranes is not diffusion-controlled. Biochim Biophys Acta. 1986 Jun 26;858(2):221–234. doi: 10.1016/0005-2736(86)90327-5. [DOI] [PubMed] [Google Scholar]
- Blackwell M. F., Gounaris K., Zara S. J., Barber J. A method for estimating lateral diffusion coefficients in membranes from steady-state fluorescence quenching studies. Biophys J. 1987 May;51(5):735–744. doi: 10.1016/S0006-3495(87)83400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Erecinska M. 12-(9-Anthroyl)stearic acid, a fluorescent probe for the ubiquinone region of the mitochondrial membrane. Eur J Biochem. 1975 Jun;54(2):521–529. doi: 10.1111/j.1432-1033.1975.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Chazotte B., Hackenbrock C. R. Lateral diffusion as a rate-limiting step in ubiquinone-mediated mitochondrial electron transport. J Biol Chem. 1989 Mar 25;264(9):4978–4985. [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Crane F. L. Isolation of Two Quinones with Coenzyme Q Activity from Alfalfa. Plant Physiol. 1959 Sep;34(5):546–551. doi: 10.1104/pp.34.5.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Fato R., Battino M., Degli Esposti M., Parenti Castelli G., Lenaz G. Determination of partition and lateral diffusion coefficients of ubiquinones by fluorescence quenching of n-(9-anthroyloxy)stearic acids in phospholipid vesicles and mitochondrial membranes. Biochemistry. 1986 Jun 3;25(11):3378–3390. doi: 10.1021/bi00359a043. [DOI] [PubMed] [Google Scholar]
- Fato R., Battino M., Parenti Castelli G., Lenaz G. Measurement of the lateral diffusion coefficients of ubiquinones in lipid vesicles by fluorescence quenching of 12-(9-anthroyl)stearate. FEBS Lett. 1985 Jan 7;179(2):238–242. doi: 10.1016/0014-5793(85)80526-3. [DOI] [PubMed] [Google Scholar]
- González-Halphen D., Lindorfer M. A., Capaldi R. A. Subunit arrangement in beef heart complex III. Biochemistry. 1988 Sep 6;27(18):7021–7031. doi: 10.1021/bi00418a053. [DOI] [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Gupte S., Wu E. S., Hoechli L., Hoechli M., Jacobson K., Sowers A. E., Hackenbrock C. R. Relationship between lateral diffusion, collision frequency, and electron transfer of mitochondrial inner membrane oxidation-reduction components. Proc Natl Acad Sci U S A. 1984 May;81(9):2606–2610. doi: 10.1073/pnas.81.9.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H., van Gelder B. F., King T. E. Preparation of cytochrome oxidase from beef heart. Methods Enzymol. 1978;53:54–66. doi: 10.1016/s0076-6879(78)53013-9. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E. N-D-Gluco-N-methylalkanamide compounds, a new class of non-ionic detergents for membrane biochemistry. Biochem J. 1982 Nov 1;207(2):363–366. doi: 10.1042/bj2070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. K., Krogmann D. W. Cytochrome f from spinach and cyanobacteria. Purification and characterization. J Biol Chem. 1980 May 10;255(9):3855–3861. [PubMed] [Google Scholar]
- Karlsson B., Hovmöller S., Weiss H., Leonard K. Structural studies of cytochrome reductase. Subunit topography determined by electron microscopy of membrane crystals of a subcomplex. J Mol Biol. 1983 Apr 5;165(2):287–302. doi: 10.1016/s0022-2836(83)80258-7. [DOI] [PubMed] [Google Scholar]
- Leonard K., Wingfield P., Arad T., Weiss H. Three-dimensional structure of ubiquinol:cytochrome c reductase from Neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol. 1981 Jun 25;149(2):259–274. doi: 10.1016/0022-2836(81)90301-6. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim Biophys Acta. 1985 Dec 19;821(3):470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- Minton A. P. Lateral diffusion of membrane proteins in protein-rich membranes. A simple hard particle model for concentration dependence of the two-dimensional diffusion coefficient. Biophys J. 1989 Apr;55(4):805–808. doi: 10.1016/S0006-3495(89)82880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman G. C., Huang C. Structural studies on phophatidylcholine-cholesterol mixed vesicles. Biochemistry. 1975 Jul 29;14(15):3363–3370. doi: 10.1021/bi00686a012. [DOI] [PubMed] [Google Scholar]
- O'Leary T. J. Lateral diffusion of lipids in complex biological membranes. Proc Natl Acad Sci U S A. 1987 Jan;84(2):429–433. doi: 10.1073/pnas.84.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink D. A., Georgallas A., Chapman D. Intrinsic proteins and their effect upon lipid hydrocarbon chain order. Biochemistry. 1981 Dec 8;20(25):7152–7157. doi: 10.1021/bi00528a015. [DOI] [PubMed] [Google Scholar]
- Rosevear P., VanAken T., Baxter J., Ferguson-Miller S. Alkyl glycoside detergents: a simpler synthesis and their effects on kinetic and physical properties of cytochrome c oxidase. Biochemistry. 1980 Aug 19;19(17):4108–4115. doi: 10.1021/bi00558a032. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Effects of impermeable patches on diffusion in a cell membrane. Biophys J. 1982 Aug;39(2):165–173. doi: 10.1016/S0006-3495(82)84504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys J. 1987 Dec;52(6):989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Meers P. R., Webb W. W. Lateral diffusion of gramicidin C in phospholipid multibilayers. Effects of cholesterol and high gramicidin concentration. Biophys J. 1982 Nov;40(2):129–135. doi: 10.1016/S0006-3495(82)84467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. A., Ravikumar K. The gramicidin pore: crystal structure of a cesium complex. Science. 1988 Jul 8;241(4862):182–187. doi: 10.1126/science.2455344. [DOI] [PubMed] [Google Scholar]
- Whitmarsh J., Ort D. R. Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys. 1984 Jun;231(2):378–389. doi: 10.1016/0003-9861(84)90401-6. [DOI] [PubMed] [Google Scholar]
- van Beijeren H, Kutner R. Mean square displacement of a tracer particle in a hard-core lattice gas. Phys Rev Lett. 1985 Jul 8;55(2):238–241. doi: 10.1103/PhysRevLett.55.238. [DOI] [PubMed] [Google Scholar]
- van Gelder B. F. On cytochrome c oxidase. I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966 Apr 12;118(1):36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]


