Abstract
Pathogenic strains of Yersinia spp. inject a set of Yop effector proteins into eukaryotic cells by using a plasmid-encoded type III secretion system. In this study, we analyzed the inflammatory response of human umbilical vein endothelial cells (HUVECs) after infection with different Yersinia enterocolitica strains. We found that both expression of intercellular adhesion molecule 1 and release of the cytokines interleukin-6 (IL-6) and IL-8 by HUVECs are downregulated in a YopP-dependent way, demonstrating that YopP plays a major role in the inflammatory response of these cells. Infection of HUVECs with several low-virulence (biotype 2, 3, and 4) and high-virulence (biotype 1B) Y. enterocolitica strains showed that biotype 1B isolates are more efficient in inhibiting the inflammatory response than low-virulence Y. enterocolitica strains and that this effect depends on the time of contact. We extended the results of Ruckdeschel et al. and found that on the basis of the presence or absence of arginine-143 of YopP (K. Ruckdeschel, K. Richter, O. Mannel, and J. Heesemann, Infect. Immun. 69:7652-7662, 2001) all the Y. enterocolitica strains used fell into two groups, which correlate with the low- and high-virulence phenotypes. In addition, we found that high-virulence strains inject more YopP into the cytosol of eukaryotic target cells than do low-virulence strains.
The genus Yersinia contains three species that are pathogenic for rodents and humans, Yersinia pestis, Yersinia pseudotuberculosis,and Yersinia enterocolitica. Although they infect their hosts via different routes and cause diseases with different severities, these three species are organotropic for lymphatic tissue, where they proliferate as extracellular pathogens. After orogastric inoculation of mice, Y. enterocolitica and Y. pseudotuberculosis gain access to the underlying lymphoid tissue (e.g., Peyer's patches) of the intestinal mucosa through M cells (18). This invasion leads to enormous recruitment of polymorphonuclear leukocytes, formation of microabscesses comprising extracellular Yersinia, and finally complete destruction of the cytoarchitecture of the Peyer's patches (1). The appearance of abscesses in mesenteric lymph nodes later in the infection process suggests that Y. enterocolitica and Y. pseudotuberculosis are disseminated via the lymphatic vessels.
To overcome the primary immune response of the host cells, yersiniae contain a sophisticated virulence system encoded by a 70-kDa virulence plasmid called pYV (Yersinia virulence) in Y. enterocolitica (7, 8) that encodes the Ysc type III secretion system. Pathogenic Y. enterocolitica strains can be divided into a low-virulence group (biotypes 2 to 4) and a high-virulence group (biotype 1B) on the basis of lethality for mice. Both groups have a functional pYV plasmid, but in addition the high-virulence group has two chromosomally encoded pathogenicity islands, one carrying genes involved in iron uptake (2, 36) and the other encoding a second type III system called the Ysa system (15, 19). The function of the Ysa type III secretion system in virulence has not been clearly established yet. The Ysc type III secretion machinery becomes activated upon contact with eukaryotic cells and directs Yops over the bacterial membranes. Some of the Yops form pores in the eukaryotic target cell membrane, while the other Yops are effector proteins that are delivered through these pores into the cytosol of the target cell. At least six different Yop effectors are injected by the Ysc secretion translocation apparatus. The effectors YopE, YopH, YopO (called YpkA in Y. pseudotuberculosis), and YopT have a negative role in cytoskeleton dynamics (for a review see reference 10). At least YopE and YopH have been shown to contribute to the strong resistance of Yersinia to phagocytosis (4). YopM has been shown to translocate to the nucleus, but its target and role remain unknown (46). One of the effectors, called YopP in Y. enterocolitica and YopJ in Y. pseudotuberculosis and Y. pestis, causes a variety of effects, such as suppression of tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8) production, blocking of activation of mitogen-activated protein kinases (MAPK) and nuclear factor κB (NF-κB) (5, 31, 33, 34, 42), and induction of apoptosis in macrophages (14, 26, 28, 38). YopJ-induced apoptosis by Y. pseudotuberculosis has been observed during an experimental mouse infection, showing that apoptosis plays a role in the establishment of a systemic infection (27). YopP/J interacts with IΚβ kinase (IKKβ) and MAPK kinases (MKKs), and recently it has been suggested that YopP/J belongs to a family of cysteine proteases related to the ubiquitin-like protein proteases (9, 31, 32). Mutation of the YopP/J-predicted catalytic cysteine-172, which presumably results in a loss of protease activity, hampers the capacity of the protein to inhibit the NF-κB and MAPK signaling cascades and to induce apoptosis (14, 32). It was recently demonstrated that besides cysteine-172, arginine-143, which is present only in high-virulence Y. enterocolitica strains and in YopJ (Y. pestis and Y. pseudotuberculosis), plays a major role in determining the inhibitory effect of YopP on the suppression of NF-κB activation and the survival of macrophages (39).
Inflammation is a process that brings cells and molecules of the immune response system to sites of infection and damage in order to clear the host of offending organisms and microbial material. In addition to macrophages and epithelial cells, endothelial cells play a key role in this process because they are involved in the recruitment of polymorphonuclear leukocytes and macrophages to the site of tissue injury. This is accomplished through expression of cellular adhesion molecules, including E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cellular adhesion molecule 1. In addition to expressing cellular adhesion molecules, endothelial cells release proinflammatory cytokines, such as IL-6 and IL-8 (24). Since they are part of the lymphatic vessels, endothelial cells are also likely to be physiological targets of Yersinia and its effectors during infection. We have recently demonstrated that Y. enterocolitica translocates Yop effectors into human umbilical vein endothelial cells (HUVECs) and causes effector-dependent cytotoxicity (6). Here, we investigated the endothelial inflammatory response after infection with different low- and high-virulence Y. enterocolitica strains. We found that infection of HUVECs with both low- and high-virulence wild-type Y. enterocolitica strains downregulates the release of IL-6 and IL-8 and the expression of the adhesion factor ICAM-1 in a YopP-dependent way. However, in low-virulence Y. enterocolitica strains these effects are dependent on the time of contact. Our results also indicate that the higher efficiency of biotype 1B high-virulence Y. enterocolitica serotypes in preventing the HUVEC inflammatory response could be correlated not only with the sequence of YopP but also with the amounts of YopP injected by the different biotypes.
MATERIALS AND METHODS
Bacterial strains, plasmids, DNA manipulations, and growth conditions.
Escherichia coli Top10 (Invitrogen) was used for standard manipulations, and E. coli SM10 lambda pir+ (25) was used to deliver mobile plasmids into Y. enterocolitica. E. coli strains were routinely grown at 37°C in tryptic soy broth or on tryptic soy agar plates containing the appropriate antibiotics. Y. enterocolitica E40(pYV40) is a wild-type, low-virulence strain belonging to serotype O:9 (Tables 1 and 2) (47). Y. enterocolitica E40(pMSK41) is a yopP knockout (yopP23 allele) derivative of E40(pYV40) (26). Y. enterocolitica A127(pYV127) is a wild-type, high-virulence strain belonging to serotype O:8 (Tables 1 and 2) (21). To construct a Y. enterocolitica A127 yopP mutant, a 482-bp BamHI DNA fragment corresponding to the internal part of yopP was first inserted at the unique BamHI site of pBluescript II KS(+) (Stratagene), yielding pILR16. A yopP mutator plasmid was then constructed by cloning a 555-bp ApaI-XbaI DNA fragment from pILR16 into pPT7, which resulted in pILC19. Plasmid pPT7 is a derivative of the pKNG101 suicide vector (23) without the PstI sites and without the sacBR operon (Troisfontaines and Cornelis, unpublished data). The Y. enterocolitica A127 yopP mutant was obtained by a single recombination event after conjugation of pILC19 into Y. enterocolitica A127, which yielded Y. enterocolitica A127(pILC127-19). For reasons of clarity, the YopP-deficient strains are referred to below as E40YopP− and A127YopP−. Plasmids pMSK13 and pSI69 are mobilizable plasmids containing yopPE40WT (from the pYV plasmid of Y. enterocolitica E40) and yopPA127WT (from the pYV plasmid of Y. enterocolitica A127), respectively. Plasmid pMSK13 has been described elsewhere (26). To obtain pSI69, yopPA127WT was PCR amplified and cloned downstream of the strong yopE promoter in the pCNR26 vector (41). Plasmids pRB16 and pILR15 are derivatives of pMSK13 and pSI69 encoding yopPE40C172T and yopPA127C172T, respectively, in which the catalytic cysteine is replaced by a threonine. The yopPA127C172T mutant present in pILR15 was obtained as previously described for pRB16 (14). Plasmids pMSK13, pSI69, pRB16, and pILR15 were used to complement the YopP deficiency in strains E40YopP− and A127YopP−. For reasons of clarity, strains E40(pMSK41)(pMSK13) and E40(pMSK41)(pRB16) are referred to below as E40YopPE40WT+++ and E40YopPE40C172T+++, respectively, and strains A127(pILC127-19)(pSI69) and A127(pILC127-19)(pILR15) are referred to below as A127YopPA127WT+++ and A127YopPA127C172T+++, respectively. Strains E40 and A127 and the derivative strains are listed in Table 1; the other Yersinia strains used in this study are listed in Table 2. DNA manipulations were performed by using standard methods (40). For sequencing of yopP, pYV plasmid DNA was purified from all pYV+ strains listed in Table 2, and PCRs were performed to amplify the yopP genes. By using four different internal primers, the yopP genes of the different strains were sequenced with a CEQ 2000 dye terminator cycle sequencing kit and a CEQ 2000 automated sequencer (Beckman Coulter). For plasmid construction, DNA fragments were purified from agarose gels by using a QIAquick kit (QIAGEN). PCRs were performed with high-fidelity Vent DNA polymerase (New England Biolabs). The nucleotide sequences of all the plasmids obtained after PCR amplification were checked by DNA sequencing by using a CEQ 2000 automated sequencer (Beckman Coulter). Y. enterocolitica strains were grown at 25°C in brain heart infusion medium (Difco) or on tryptic soy agar plates containing the appropriate antibiotics. Prior to use, Y. enterocolitica strains were pregrown overnight at 25°C with continuous shaking (110 rpm). For infection, bacteria were then diluted to an optical density of 0.1 in fresh brain heart infusion medium and cultured under the same conditions for 120 min. Subsequently, Yop secretion was induced by incubation for 30 min in a shaking water bath (110 rpm) at 37°C. Prior to infection bacteria were washed with basic endothelial growth medium (bEGM) (PromoCell, Heidelberg, Germany). For in vitro Yop secretion, Y. enterocolitica strains were diluted to an optical density of 0.1 and grown with shaking at room temperature in brain heart infusion medium supplemented with 20 mM sodium oxalate, 20 mM MgCl2, and 0.4% glucose. After 2 h, the cultures were transferred to a 37°C water bath and incubated for 4 h with shaking.
TABLE 1.
Y. enterocolitica E40 and A127 and derivative strains
| Strain designation | Strain | Relevant characteristics | Reference |
|---|---|---|---|
| E40 | 47 | ||
| E40pYV+ | E40(pYV40) | Wild-type E40 | |
| E40pYV− | E40(pYV40)− | E40 cured of its pYV plasmid | |
| E40YopP− | E40(pMSK41) | yopP knockout (yopP23 allele) of E40(pYV40) | 26 |
| E40YopPE40WT+++ | E40(pMSK41)(pMSK13) | E40(pMSK41), complemented with pMSK13, a multicopy plasmid overexpressing wild-type YopPE40 | 14 |
| E40YopPE40C172T+++ | E40(pMSK41)(pRB16) | E40(pMSK41), complemented with pRB16, a plasmid overexpressing catalytically inactive YopPE40 | 14 |
| A127 | 21 | ||
| A127pYV+ | A127(pYV127) | Wild-type A127 | |
| A127pYV− | A127(pYV127)− | A127 cured of its pYV plasmid | |
| A127YopP− | A127(pILC127-19) | yopP knockout (yopP23 allele) of A127(pYV127) | This study |
| [A127(pILC127-19) = pYV127yopP23] | |||
| A127YopPA127WT+++ | A127(pILC127-19)(pSI69) | A127(pILC127-19), complemented with pSI69, a plasmid overexpressing wild-type YopPA127 | This study |
| (pSI69 is pCNR26 containing yopPA127 cloned in NdeI and HindIII sites downstream the yopE promoter) | |||
| A127YopPA127C172T+++ | A127(pILC127-19)(pILR15) | A127(pILC127-19), complemented with pILR15, a plasmid overexpressing catalytically inactive YopPA127 | This study |
| (pILR15 is pCNR26 containing yopPA127C172T, obtained by site-directed mutagenesis of pSI69) |
TABLE 2.
Y. enterocolitica clinical isolates
| Strain | Serotype | Origin | Year of isolation | Reference or source | |
|---|---|---|---|---|---|
| Biotype 2 strains
|
|||||
| E40 | O:9 | Belgium | 1995 | 47 | |
| W22703 | O:9 | Belgium | 1978 | 48 | |
| W1024 | O:9 | Belgium | 1988 | 13 | |
| WA210/99 | O:9 | Belgium | 1999 | G. Wauters | |
| Biotype 3 strain
|
|||||
| AT3P1 | O:1 | Canada | G. Wauters | ||
| Biotype 4 strains
|
|||||
| W804 | O:3 | Belgium | G. Wauters | ||
| C1423 | O:3 | 1992 | G. Wauters | ||
| WA234/99 | O:3 | Belgium | 1999 | G. Wauters | |
| WA235/99 | O:3 | Belgium | 1999 | G. Wauters | |
| WA236/99 | O:3 | Belgium | 1999 | G. Wauters | |
| Biotype 1B strains
|
|||||
| WAQ70 | O:4 | United States | 1972 | G. Wauters | |
| A127/90 | O:8 | Japan | 1990 | 21 | |
| 8081 | O:8 | United States | 35 | ||
| WAT297 | O:8 | United States | 1983 | G. Wauters | |
| A285 | O:13a O:13b | Canada | 1986 | G. Wauters | |
| WAT2567 | O:13 | Canada | 1984 | G. Wauters | |
| T4786 | O:13 | Canada | 1982 | G. Wauters | |
| A11/86 | O:21 | Canada | 1986 | G. Wauters | |
Isolation and growth of HUVECs.
HUVECs were isolated by using umbilical cords from healthy donors essentially as described by Jaffe et al. (22). In brief, veins were washed with phosphate-buffered saline (PBS) prewarmed to 37°C before incubation with 0.1% (wt/vol) collagenase (Sigma) in PBS for 30 min at 37°C. The collagen solution was collected from the veins by washing with bEGM and was centrifuged for 10 min at 350 × g. The pelleted cells were resuspended in bEGM supplemented with 10% heat-inactivated fetal bovine serum, endothelial cell growth supplement-heparin (0.4%), epidermal growth factor (0.1 ng/ml), basic fibroblast growth factor (1 ng/ml), hydrocortisone (1 ng/ml), streptomycin (100 μg/ml), penicillin (100 U/ml), and amphotericin B (2.5 μg/ml) and incubated overnight in gelatin-coated culture flasks at 37°C in a humidified atmosphere containing 6% CO2. Erythrocytes and nonadherent cells were washed off the monolayer with fresh medium, and cells were grown for up to six passages on gelatin-coated surfaces.
Cell culture and infection.
Murine monocyte-macrophage J774A.1 cells (ATCC TIB67) and human epithelial HeLa cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin sulfate (100 μg/ml), Na pyruvate (1 mM), and β-mercaptoethanol (2 × 10−5 M); Rat-1 fibroblasts were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin sulfate (100 μg/ml), and Na pyruvate (1 mM); and HUVECs were cultured in complete bEGM. Cells were cultured at 37°C under 6% CO2. Prior to infection cells were seeded into medium without antibiotics. After 15 h, the cells were infected with the relevant Y. enterocolitica strains grown under conditions for moderate Yop induction at 37°C (see above) by using a multiplicity of infection (MOI) of 50. Extracellular bacteria were killed 1 or 3 h after infection by adding gentamicin (50 μg/ml).
IL-6 and IL-8 enzyme-linked immunosorbent assays (ELISA).
At 5 or 24 h after the end of a 1- or 3-h infection, cell supernatant was removed to determine IL-6 and IL-8 concentrations with BIOTRAK human IL-8 and IL-6 assay kits (Amersham Pharmacia Biotech), as described by the manufacturer. All results presented are based on three independent experiments.
Statistical analysis.
We used analysis of variance (ANOVA) for two different purposes. First, we tested whether the presence or absence of YopP led to differences in induction of cytokine release (for the results of this analysis see Fig. 1 and 4). Each of the eight combinations of exposure time (1 and 3 h), genetic background (E40 and A127), and cytokine (IL-6 and IL-8) was tested with a two-way ANOVA with the factors presence or absence of YopP (fixed, with the levels noninfected, pYV−, pYV+, YopP−, YopPWT+++, and YopPC172T+++) and experiment (random, with the factors exp. 1 to exp. 3). As we specifically wanted to analyze which strains differed from a corresponding pYV− strain, we used a post hoc Dunnett's pairwise multiple comparison t test to compare the set of strains against a single control mean, the pYV− strain. This test adjusted the significance level for a fivefold comparison within each of the eight combinations that were tested. As a preliminary analysis revealed that the residuals were not normally distributed, all values were log transformed prior to analysis to reduce the deviation from normality. Second, we tested whether the effect of the presence of the pYV plasmid during induction of IL-8 release differed for low- and high-virulence biotypes (for the results of this analysis see Fig. 2). This was done with a three-way ANOVA with the factors pYV (fixed, with the levels pYV+ and pYV−), serotype (fixed, with the levels high virulence and low virulence), and isolate (random, nested in serotype). A difference in the effect of the presence of pYV between serotypes was manifested as a statistical interaction between serotype and pYV. All ANOVAs were carried out with SPSS (release 10.1.3) procedure glm.
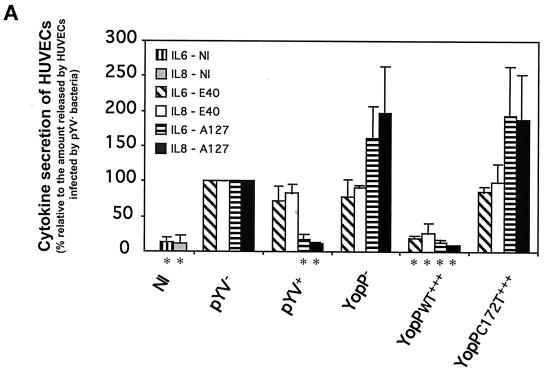
FIG. 1.
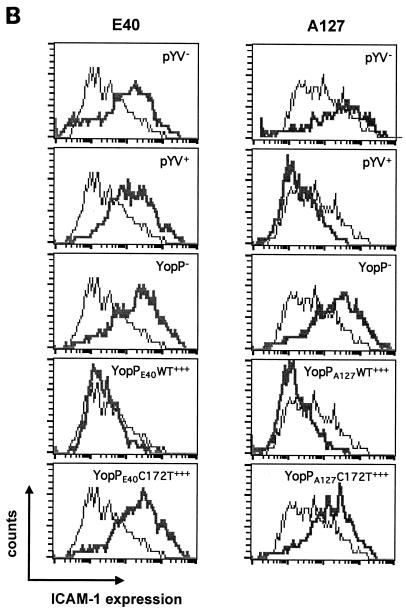
Y. enterocolitica A127 is more efficient than Y. enterocolitica E40 in downregulating the inflammatory response of HUVECs. HUVECs were infected at an MOI of 50 with the following derivatives of Y. enterocolitica E40 and A127: wild type (pYV+), pYV plasmid cured (pYV−), YopP deficient (YopP−), YopP− overexpressing the wild-type YopP (YopPWT+++), and YopP− overexpressing the catalytically inactive mutant (YopPC172T+++) (Table 1; also see Materials and Methods). Noninfected (NI) HUVECs were used as a control. After 1 h of infection, gentamicin (50 μg/ml) was added to kill extracellular bacteria. (A) IL-6 secretion and IL-8 secretion analyzed by ELISA in cell culture supernatants collected 5 h after the beginning of the infection. An asterisks below a bar indicates that the cytokine secretion value for the strain was significantly different from the value for the corresponding pYV strain (Dunnett's post hoc analysis, P < 0.05) (see Materials and Methods). (B) ICAM-1 expression monitored by flow cytometry 24 h after the beginning of infection with mouse monoclonal antibodies against ICAM-1 conjugated to fluorescein. Thin lines, ICAM-1 expression of noninfected HUVECs; thick lines, ICAM-1 expression of infected HUVECs.
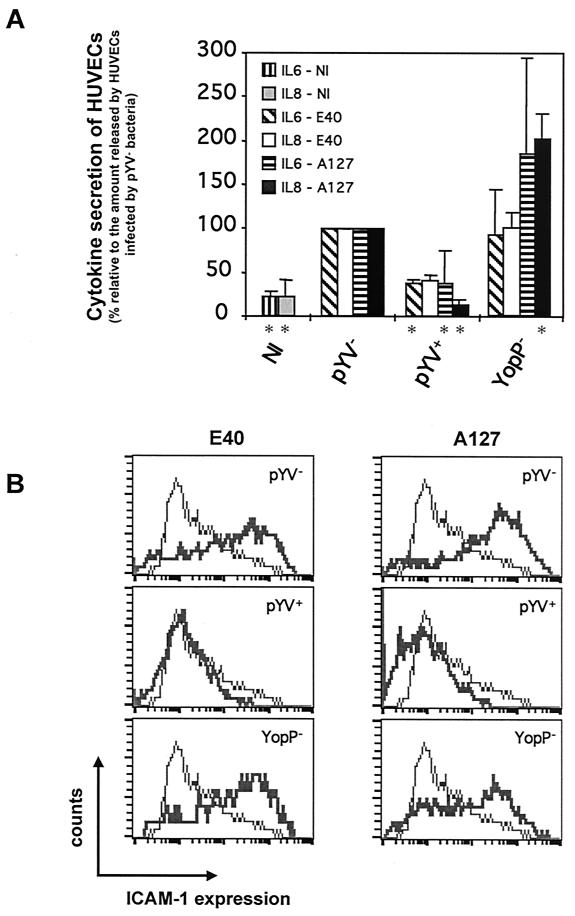
FIG. 4.
Inhibition of the release of anti-inflammatory cytokines and the expression of ICAM-1 adhesion molecules by HUVECs infected by Y. enterocolitica for 3 h. HUVECs were infected at an MOI of 50 with the following derivatives of Y. enterocolitica E40 and A127: wild type (pYV+), pYV plasmid cured (pYV−), and YopP deficient (YopP−) (Table 1; also see Materials and Methods). Noninfected (NI) HUVECs were used as a control. After 3 h of infection, gentamicin (50 μg/ml) was added to kill extracellular bacteria. (A) IL-6 secretion and IL-8 secretion were analyzed by ELISA by using cell culture supernatants collected 5 h after the beginning of the infection. An asterisk below a bar indicates that the cytokine secretion value for the strain was significantly different from the value for the corresponding pYV− strain (Dunnett's post hoc analysis, P < 0.05) (see Materials and Methods). (B) ICAM-1 expression monitored by flow cytometry 24 h after infection with mouse monoclonal antibodies against ICAM-1 conjugated to fluorescein. Thin lines, ICAM-1 expression of noninfected HUVECs; thick lines, ICAM-1 expression of infected HUVECs.
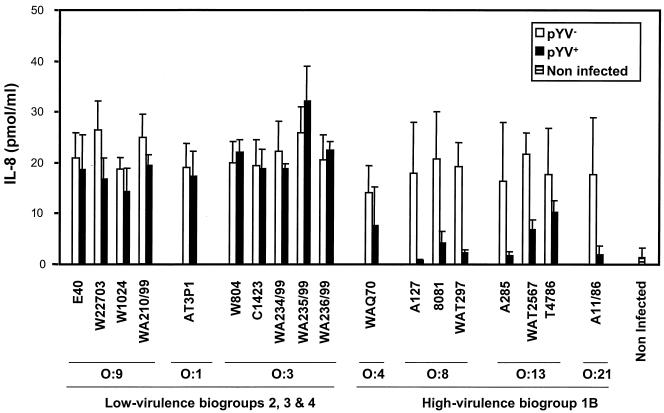
FIG. 2.
Suppression of IL-8 release is dependent on the Y. enterocolitica serotype. HUVECs were infected with Y. enterocolitica strains and their corresponding pYV− derivatives (Table 2) at an MOI of 50. Noninfected HUVECs were used as a control. After 1 h of infection, gentamicin (50 μg/ml) was added to kill extracellular bacteria. The amount of IL-8 released was assayed by using cell supernatants collected 24 h after the beginning of infection as described in the legend to Fig. 1. The ANOVA revealed a significant interaction between serotype and pYV (df = 1, MS = 896.8, F = 29.4, P < 0.001), supporting the conclusion that suppression of IL-8 release depends on the serotype.
Flow fluorocytometric analysis of the adhesion molecule ICAM-1.
Twenty-four hours after the end of a 1- or 3-h infection, cells were harvested and resuspended in HAFA buffer (137 mM NaCl, 5 mM KCl, 0.3 mM Na2HPO4, 0.7 mM KH2PO4, 0.4 mM MgSO4, 0.3 mM MgCl2, 5 mM glucose, 6 mM NaHCO3, 1 mM EDTA, 3% [wt/vol] fetal bovine serum, 20 mM NaN3) to obtain 2 × 105 cells in 200 ml. The cell suspensions were incubated for 1 h at 4°C with anti-human mouse monoclonal antibodies against ICAM-1, directly conjugated to fluorescein (R&D Systems). After the cells were washed with PBS, they were analyzed with a FACSCAN flow fluorescence cytometer (Becton-Dickinson).
Western analysis of Yops.
Five hours after the end of a 1- or 3-h infection, cells were harvested, washed with PBS, resuspended in 0.02% digitonin (Calbiochem), and incubated for 3 min on ice. Cell membranes and organelles were pelleted by centrifugation (15,000 × g for 5 min at 4°C). Subsequently, cytosolic fractions from an equivalent of 2 × 105 cells were loaded on sodium dodecyl sulfate-12% polyacrylamide gels. After electrophoresis, the gels were blotted onto nitrocellulose (BA 83; Schleicher & Schuell), which was then probed with a rabbit polyclonal anti-YopP antiserum (14) or a rabbit polyclonal anti-YopH antiserum. Primary antibody binding was detected with horseradish peroxidase-conjugated swine anti-rabbit immunoglobulin G (PROSAN) and was visualized by chemiluminescence by using a Renaissance kit (NEN, Life Science Products) as recommended by the manufacturer.
RESULTS
YopP of both low- and high-virulence Y. enterocolitica strains is able to downregulate the release of the proinflammatory cytokines IL-6 and IL-8 by HUVECs.
To investigate the inflammatory response of endothelial cells towards Y. enterocolitica, we analyzed both IL-6 secretion and IL-8 secretion after infection with a low-virulence Y. enterocolitica strain (E40) and after infection with a high-virulence Y. enterocolitica strain (A127) (Tables 1 and 2). To do this, HUVECs were initially infected with wild-type Y. enterocolitica (pYV+) and the pYV plasmid-cured variants (pYV−) of strains E40 and A127. Bacteria were killed with gentamicin 1 h after infection to allow injection of Yop effectors for 1 h, and the release of IL-6 and IL-8 was monitored 6 h after the beginning of the infection by using ELISA. Importantly, in these experimental conditions no significant differences were observed between the levels of IL-6 and IL-8 release in HUVECs infected with the low-virulence E40pYV+ strain and the levels of IL-6 and IL-8 release in HUVECs infected with the E40pYV− strain, while there was clear inhibition of cytokine secretion when HUVECs were infected with the A127pYV+ strain compared to the secretion when HUVECs were infected with the plasmid-cured strain (Fig. 1A). These results show that when bacteria are allowed to inject Yops for 1 h, the release of IL-6 and IL-8 by HUVECs is downregulated when a high-virulence Y. enterocolitica strain is used but not when a low-virulence strain is used.
YopP has been shown to decrease the secretion of cytokines in other infection models (5, 33, 42). To demonstrate that YopP is responsible for the observed negative effect on the inflammatory response of HUVECs after Y. enterocolitica A127pYV+ infection, A127YopP−, a mutant strain deficient in the YopP effector, was used (Table 1; also see Materials and Methods). Infection of HUVECs with the A127YopP− strain resulted in even higher levels of IL-6 and IL-8 release than infection of HUVECs with A127pYV− (Fig. 1A). Furthermore, when we infected HUVECs with a Y. enterocolitica A127 derivative in which the YopP deficiency is complemented by a plasmid overexpressing wild-type YopP (A127YopPA127WT+++), the levels of cytokine release were identical to those detected in pYV+-infected cells (Fig. 1A). We also infected HUVECs with an A127YopP− derivative carrying a plasmid that expresses a catalytically inactive mutant of YopP (A127YopPA127C172T+++). In this case, the levels of cytokine secretion were similar to those observed with the A127YopP− strain (Fig. 1A). In parallel, HUVECs were infected with the E40 YopP-deficient strain (E40YopP−) and with the E40YopP− derivatives carrying plasmids overexpressing wild-type YopP (E40YopPE40WT+++) and its catalytically inactive mutant (E40YopPE40C172T+++). Infection of HUVECs with the E40YopP− strain resulted in no difference in the levels of cytokine release compared to the levels after infection of HUVECs with E40pYV− (Fig. 1A). However and importantly, infection with the Y. enterocolitica E40YopPE40WT+++ strain resulted in clear inhibition of the inflammatory response of HUVECs (Fig. 1A). Therefore, although the Y. enterocolitica E40pYV+ strain was unable to downregulate the endothelial inflammatory response in these conditions, overexpression of YopP from E40 in a yopP background resulted in inhibition of IL-6 and IL-8 release. Infection experiments with the E40YopPE40C172T+++ strain resulted in levels of cytokine secretion similar to those observed with the E40YopP− strain (Fig. 1A), which eliminated the possibility that the latter effect was merely a consequence of the overproduction of a Yop effector. In addition, overexpression of other Yop proteins (e.g., YopO) did not result in suppression of the release of proinflammatory cytokines (data not shown). These data confirmed that YopP is indeed the effector responsible for inhibition of cytokine release after Y. enterocolitica infection of endothelial cells and that the putative proteolytic activity of YopP is required for the observed downregulation. Furthermore, our results indicate that YopP from the high-virulence Y. enterocolitica A127 strain is more efficient than YopP from the low-virulence E40 strain in preventing the HUVEC inflammatory response. However, it is also clear that when YopP from the low-virulence E40 strain is overexpressed, it has the same effect as YopP from the high-virulence A127 strain.
YopP prevents expression of the cellular adhesion molecule ICAM-1.
To further study the ability of wild-type Y. enterocolitica to inhibit the inflammatory response after HUVEC infection, we next examined the expression of ICAM-1, a cellular adhesion molecule that plays an important role in inflammation. A time kinetic analysis showed that expression of ICAM-1 by HUVECs, as measured by flow cytometry, is maximal 24 h after infection with Y. enterocolitica E40pYV− (data not shown). Therefore, we analyzed the expression of ICAM-1 at that time point after infection with the low- and high-virulence Yersinia strains used to study IL-6 and IL-8 release (see above). As before, the bacteria were killed 1 h after infection. Compared to expression in pYV−-infected cells, ICAM-1 expression was strongly inhibited in endothelial cells infected with the A127pYV+ strain, but it was not affected in HUVECs infected with the E40pYV+ strain (Fig. 1B). Infection of HUVECs with the A127YopP− or E40YopP− strain did not result in inhibition of ICAM-1 expression (Fig. 1B). When the A127YopPA127WT+++ and E40YopPE40WT+++ strains were used, a clear reduction in adhesin expression was observed (Fig. 1B), confirming that when YopPE40 is overexpressed, it is as functional as YopPA127. Finally, infection of HUVECs with the A127YopPA127C172T+++ and E40YopPE40C172T+++ strains had an effect on ICAM-1 expression similar to that of the corresponding YopP-deficient strains, A127YopP− and E40YopP− (Fig. 1B). These results demonstrated that YopP, through its putative proteolytic activity, also inhibits ICAM-1 expression after Yersinia infection of HUVECs.
Effect of Y. enterocolitica on the HUVEC inflammatory response is serotype dependent.
In order to analyze if the differential action of the E40 and A127 strains on the HUVEC inflammatory response is linked to the Y. enterocolitica serotype or is just due to individual variations between strains, we infected HUVECs with various wild-type isolates belonging to different serotypes and their corresponding pYV− variants (Table 2). To do this, bacteria were killed with gentamicin 1 h after infection, and the release of IL-8 was monitored as described above. Infection with pYV+ strains of different high-virulence Y. enterocolitica serotypes (serotypes O:4, O:8, O:13, and O:21) resulted in 3- to 38-fold reductions in IL-8 release compared to the IL-8 release after infection with the corresponding isogenic pYV− strains (Fig. 2). Importantly, infection with the pYV+ low-virulence Y. enterocolitica strains belonging to serotypes O:9, O:3, and O:1 did not lead to inhibition of cytokine release compared to the cytokine release observed after infection with the corresponding pYV− variants (Fig. 2). These data show that the higher efficiency displayed in downregulation of the HUVEC inflammatory response by the high-virulence Y. enterocolitica A127 strain is a characteristic that is intrinsic to all high-virulence Y. enterocolitica serotypes.
YopP sequences of the different low- and high-virulence Y. enterocolitica strains.
IL-6 and IL-8 production and ICAM-1 expression are known to be NF-κB dependent (24). While this work was in progress, Ruckdeschel et al. (39) reported that arginine-143 of YopP from high-virulence strain WA-314 crucially determines the efficiency of the inhibitory impact of YopP on NF-κB activity and the survival of macrophages. Accordingly, our observation of the different efficiencies displayed by YopP proteins from Y. enterocolitica low- and high-virulence strains might be partially explained at the level of the amino acid sequence of YopP. Therefore, we sequenced yopP from all the low- and high-virulence Y. enterocolitica strains used in this work (Table 2). The nucleotide sequences of the nine yopP genes from the low-virulence strains are 100% identical to the previously published sequence of Y. enterocolitica W22703 (GenBank accession no. NC_002120.1). Moreover, the DNA sequences of the yopP genes from the eight high-virulence strains (serotypes O:4, O:8, O:13, and O:21) are 100% identical to the previously published sequence of Y. enterocolitica 8081 (GenBank accession no. AF336309.1) except for one nucleotide (position 821 [T → C]) which differs in three of the strains (A127, A11/86, and WAT297). When the yopP DNA sequence from the high-virulence strains is compared with the yopP sequence from the low-virulence strains, there are 24 nucleotides that differ. Of the 24 nucleotides that differ, 16 cause a change in the amino acid sequence. Importantly, the arginine at position 143 occurs in all high-virulence strains, while all low-virulence strains have a serine at this position (Fig. 3). On the other hand, the putative catalytic triad which is crucial for the activity of YopP is conserved in all low- and high-virulence Y. enterocolitica strains (Fig. 3). Hence, the deduced YopP amino acid sequences of all the strains analyzed revealed two groups of YopPs that correlate with the low- and high-virulence phenotypes.
FIG. 3.
Sequence comparison of YopP proteins from the different low- and high-virulence Y. enterocolitica strains: alignment of the conserved putative catalytic domains and the arginine/serine-143 of YopP from all the Y. enterocolitica strains listed in Table 1, as well as the sequences of YopJ from Y. pestis (accession no. NP047569) [yopjkym5 (pestis)] and YopJ from Y. pseudotuberculosis (accession no. AAA68488) [YopJypiii (pseudo)]. The arrow indicates arginine/serine-143, and the asterisks indicate the amino acid residues of the catalytic triad (histidine-109, glutamate-128, and cysteine-172).
Time dependence of YopP-induced downregulation of the inflammatory response in HUVECs.
Although it is clear that there are important amino acid differences between YopPs from the low- and high-virulence Y. enterocolitica strains, determining the anti-inflammatory capacity, our results also indicate that overexpressed YopP from the low-virulence E40 strain has the same activity as YopP from high-virulence strain A127. Therefore, we next examined whether the time that bacteria are allowed to inject Yops into the cytosol of HUVECs could be important for downregulation of the inflammatory response. We repeated the analysis of IL-6 or IL-8 release and ICAM-1 expression after Yersinia infection of HUVECs, but the bacteria were killed with gentamicin 3 h after infection rather than 1 h after infection. Interestingly, infection of HUVECs with both the E40pYV+ and A127pYV+ strains resulted in decreases in the levels of cytokine release and ICAM-1 expression compared to the levels in pYV−-infected cells (Fig. 4). Infection of HUVECs with the A127YopP− or E40YopP− strain resulted in levels of cytokine release and ICAM-1 expression similar to those observed with HUVECs infected with the pYV− strains (Fig. 4). Thus, when allowed to inject YopP for sufficient time, the low-virulence strain has the same inhibitory effect on the inflammatory response of HUVECs as the high-virulence strain.
High-virulence Y. enterocolitica strains inject more YopP into the cytoplasm of different cell types than do low-virulence Y. enterocolitica strains.
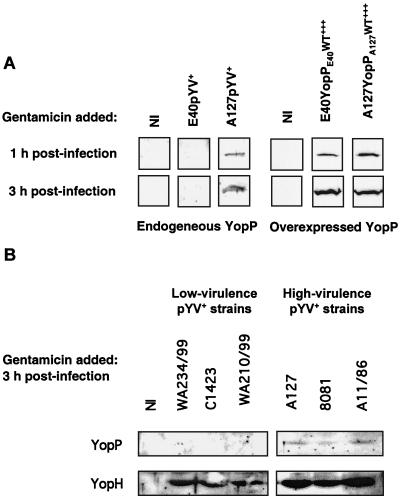
As the amount of YopP injected into the cytosol seemed to be important for its activity, we next investigated whether the amounts of endogenous YopP injected into the cytosol of HUVECs by low- and high-virulence Y. enterocolitica strains differ. Therefore, cytosolic cell lysates were prepared from HUVECs infected with the E40 and A127 pYV+ strains. We were unable to detect YopP injected by the E40 pYV+ strain, whether bacteria were killed 1 or 3 h after infection, although the antiserum was raised against YopPE40. On the other hand, YopP injected by the A127pYV+ strain could be immunodetected in the cytosolic fraction of HUVECs in the same experimental conditions (Fig. 5A). As a positive control, we also analyzed the amounts of YopP injected by E40YopPE40WT+++ and A127YopPA127WT+++, the YopP-overexpressing strains. As shown in Fig. 5A, the two strains injected equal amounts of YopP into the cytoplasm of HUVECs when bacteria were killed 1 or 3 h after infection. These results clearly indicate that the high-virulence A127pYV+ strain injects more YopP into the HUVEC cytosol than the low-virulence E40pYV+ strain injects. To eliminate the possibility that this observation was just due to individual variation between strains, we next compared the injection of YopP by different low- and high-virulence strains. HUVECs were infected with wild-type isolates belonging to different serotypes, the bacteria were killed with gentamicin 3 h after infection, and cytosolic cell lysates were made 2 h later. While we could detect YopP injected by the different pYV+ high-virulence Y. enterocolitica strains (A127, 8081, and A11/86 [Table 2]), we could not detect YopP injected by the pYV+ low-virulence Y. enterocolitica strains (WA234/99, C1423, and WA210/99 [Table 2]) (Fig. 5B). As a control, injection of another Yop effector, YopH, was also analyzed. As shown in Fig. 5B, injection of this Yop effector was apparently the same for all low- and high-virulence strains.
FIG. 5.
Differential injection of YopP proteins of low- and high-virulence Y. enterocolitica strains into the cytoplasm of HUVECs. HUVECs were infected with different Y. enterocolitica strains at an MOI of 50 (Tables 1 and 2; also see Materials and Methods). Noninfected (NI) HUVECs were used as a control. After 1 or 3 h of infection, gentamicin (50 μg/ml) was added to kill extracellular bacteria. Cytosolic cell lysates of HUVECs were prepared by digitonin lysis 5 h after the beginning of the infection and were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting by using an anti-YopP antibody or an anti-YopH antibody, as indicated. For reasons of clarity the Western blots shown for detection of overexpressed YopP are short-term exposures, while the Western blots for detection of endogenous YopP are long-term exposures. (A) E40pYV+, A127pYV+, E40YopPE40WT+++, and A127YopPA127WT+++; (B) WA234/99pYV+, C1423pYV+, WA210/99pYV+, A127pYV+, 8081pYV+, and A11/86pYV+.
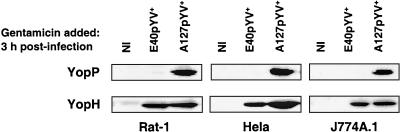
Next, we examined whether the observed difference in the levels of injection of YopP between the low- and high-virulence E40 and A127 pYV+ strains was cell type dependent. Different cell types, including fibroblasts (Rat-1), epithelial cells (HeLa), and macrophages (J774A.1), were infected with E40pYV+ or A127pYV+, and both YopP injection and YopH injection into the cytosol of these cells were analyzed. Again, bacteria were killed with gentamicin 3 h after infection, and cytosolic cell lysates were prepared 2 h later. For all three cell types, we could detect YopP from the high-virulence A127pYV+ strain but not YopP from the low-virulence E40pYV+ strain, while YopH was injected equally with both low- and high-virulence strains (Fig. 6). These data indicate that differential injection of YopP by low- and high-virulence strains is a general and specific feature, as a difference was observed with different cell types and injection of YopP was distinct from injection of YopH, which was the same for both low- and high-virulence strains.
FIG. 6.
Injection of YopP and YopH into the cytoplasm of different cell types. Rat-1, HeLa, or J774A.1 cells were infected with E40pYV+ or A127pYV+ at an MOI of 50. Noninfected (NI) cells were used as a control. After 3 h of infection, gentamicin (50 μg/ml) was added to kill extracellular bacteria. Cytosolic cell lysates of were prepared by digitonin lysis 5 h after the beginning of the infection and were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting by using an anti-YopP antibody or an anti-YopH antibody.
DISCUSSION
Previous studies of the interaction between Y. enterocolitica and macrophages or epithelial cells revealed that the Yop virulon mediates marked repression of the inflammatory response (5, 33, 37, 42, 44). Since endothelial cells lining the lymphatic vessel cells could play a role during Yersinia invasion of the lymphatic tissues, we extended the previous studies and analyzed the effect of Y. enterocolitica on the inflammatory response mediated by endothelial cells. We demonstrate here that both expression of the adhesion molecule ICAM-1 and release of the cytokines IL-6 and IL-8 by HUVECs upon infection with Y. enterocolitica are downregulated in a YopP-dependent way. Hence, the results presented in this paper, together with the results of previous experiments with macrophages and epithelial cells carried out with Yersinia yopP/J mutant strains (5, 37, 42), strongly suggest that YopP/J is a player in the downregulation of the inflammatory response upon Yersinia infection. Since IL-6 and IL-8 release and ICAM-1 expression are known to be dependent on NF-κB activation, our results are consistent with previous reports showing that YopP prevents TNF-α release, playing an anti-inflammatory role by preventing NF-κB activation (31, 37, 42). However, two other Yersinia proteins encoded by the pYV plasmid, LcrV and YopB, have been implicated in the suppression of TNF-α production during infection (3, 29, 30, 43). For LcrV it was even recently demonstrated that LcrV-induced IL-10 release could inhibit TNF-α production in zymosan A-stimulated macrophages (45). According to the currently accepted type III secretion-translocation model (11), YopB and LcrV are part of the translocation machinery that delivers Yop effectors into the eukaryotic cell, but this does not eliminate the possibility that these molecules also play a role on their own, independent of the rest of the injectisome. Although this role is now well documented, especially for LcrV, the molecular mechanism remains unknown. Therefore, Yersinia possesses different elements which have the capacity to inhibit TNF-α production and hence downregulate the inflammatory response. The relevance of the anti-inflammatory role played by these different elements during infection is a matter for future in vivo studies. Interestingly, the HUVEC inflammatory response to Y. enterocolitica infection was consistently greater with the yopP mutant strains than with the pYV− strains. One possible explanation for this is that an unknown pYV-encoded factor or simply the translocation process leads towards NF-κB activation.
When wild-type Y. enterocolitica was allowed to inject Yops into HUVECs for only 1 h, we observed that the low-virulence E40pYV+ strain (biotype 2) could not block cytokine secretion and adhesion molecule expression, whereas the high-virulence A127pYV+ Y. enterocolitica strain (biotype 1B) could do this. Importantly, the observed different effects were not restricted to the Y. enterocolitica E40 and A127 strains. When using various isolates belonging to different serotypes, we observed that none of the low-virulence Y. enterocolitica strains (biotypes 2 to 4) was able to inhibit the inflammatory response when bacteria were killed after 1 h, while all the high-virulence strains (biotype 1B) could inhibit this response. Thus, in these conditions the efficiency of inhibition of the HUVEC inflammatory response was dependent on the biotype of the Yersinia strain used for infection. If the finding that cytokine secretion and adhesion molecule expression depend on activation of NF-κB by IKKβ is taken into account, these results are in agreement with the recent observations of Ruckdeschel et al., who demonstrated that YopP from the low-virulence E40 strain is less efficient in inhibiting IKKβ activities than YopP from the high-virulence WA-314 strain (39). These authors showed that arginine-143 of YopP from the high-virulence WA-314 strain was crucially involved in both the suppression of the NF-κB pathway and the induction of apoptosis in macrophages. Replacement of arginine-143 with a serine residue, as occurs in the wild-type low-virulence E40 strain, resulted in lower functionality of YopP (39). In line with these results, sequencing of YopP from all the strains used in the present work revealed that the two groups (high and low virulence) could indeed be differentiated on the basis of the presence of arginine-143. Another region of YopP/J that has been shown to be important for the function of YopJ from Y. pseudotuberculosis is the src homology 2 (SH2)-like domain (42). Mutations in the SH2-like domain destroy the function of YopJ (42). Our sequencing analysis revealed that valine-54 and lysine-55 located in the SH2-like domain present in YopP from high-virulence Y. enterocolitica strains are replaced by an isoleucine and a glutamate in YopP from low-virulence Y. enterocolitica strains. The latter sequence does not result in an SH2-like domain when the sequence is scanned against the Pfam protein family database (http://pfam.wustl.edu/hmmsearch.shtml). It seems reasonable to hypothesize that the SH2-like domain could also contribute to the higher anti-inflammatory capacity displayed by YopP from high-virulence Y. enterocolitica strains. Despite these differences, the catalytic triad (His, Glu, Cys) is conserved in both pathogenic groups, meaning that YopPs from both the low- and high-virulence strains have the same putative cysteine protease activity and hence the same activity with their target.
Interestingly, we observed that when the low-virulence Y. enterocolitica E40 strain was allowed to inject more YopP due to overexpression of YopPE40WT in an E40YopP− background, the inflammatory response of HUVECs was inhibited, indicating that if YopP from the E40 strain is injected in higher amounts, it has the same effect as YopPA127WT. Moreover, when the low-virulence wild-type E40 strain was allowed to inject YopP for a longer time period (3 h), we observed clear inhibition of the inflammatory response of HUVECs. This further indicates that when endogenous YopP from E40pYV+ can accumulate in sufficient amounts in the cytosol of the eukaryotic cell, due to prolonged time of contact, it is as effective as YopP from the high-virulence A127pYV+ strain.
As for the in vivo relevance of our findings, our results and those of Ruckdeschel et al. (39) suggest that the pYV plasmid and a Yop effector could play a role in the high-virulence phenotype of serotypes O:4, O:8, O:13, and O:21. However, so far all the experimental evidence suggests that the difference in virulence is due to the presence of a high-pathogenicity island on the chromosome. This island encodes an iron uptake system mediated by the siderophore yersiniabactin (2, 36). Furthermore, exchanging the pYV plasmid between a low-virulence strain and a high-virulence strain does not significantly alter the mouse lethality of the strains (16, 20). In addition, preliminary results suggest that the A127YopP− strain is not attenuated in the mouse model for Yersinia infection; there is no significant difference in the oral 50% lethal dose (LD50) between this strain and wild-type Y. enterocolitica A127 (data not shown). Other authors have reported that the LD50 for a mouse of a yopJ mutant of Y. pseudotuberculosis or Y. pestis is not increased (17, 49). In contrast, Monack et al. (27) observed that a YopJ deficiency in Y. pseudotuberculosis increased the LD50 for a mouse by almost 100-fold. The same authors showed that there are more apoptotic macrophages in mice infected with wild-type Y. pseudotuberculosis than in mice infected with YopJ-deficient Y. pseudotuberculosis. Also, a subtle virulence phenotype was observed with two different yopP mutant Y. enterocolitica O:8 strains (12). These contrasting results await further investigation in order to clarify the in vivo role of YopP/J during Yersinia infection.
In conclusion, we have shown that the Y. enterocolitica YopP effector downregulates the inflammatory response of endothelial cells, suggesting that YopP/J is an important Yersinia effector possibly involved in the strong inhibition of TNF-α and gamma interferon release that is observed in infected mice (29). This indicates that YopP/J may have potential as an anti-inflammatory agent. Furthermore, our results indicate that both the sequence of YopP and the amount of YopP injected into a eukaryotic cell are important for the efficiency of downregulation of the inflammatory response of HUVECs. It is, however, intriguing that YopP activity is tuned down in two different ways in the low-virulence strains. This may have two different interpretations; either YopP is not essential during infection and hence its function may be lost, or alternatively, its contribution is important for virulence but detrimental for survival and propagation of the bacterium in the long term. In other words, low-virulence strains might have a selective advantage.
Acknowledgments
We thank D. Desnoeck for excellent technical assistance. We are very grateful to K. Thomas and the staff of the maternity ward at St. Luc Hospital (Brusssels, Belgium) for their support in obtaining umbilical cords. We also thank Geraldine Flynn (Open University, Milton Keynes, United Kingdom) for advice on the isolation of HUVECs and A. Tonon (Ludwig Institute for Cancer Research, Brussels, Belgium) for assistance with the flow cytometer. We thank G. Wauters for kindly providing several Y. enterocolitica strains.
G.D. and S.T. contributed equally to this work.
S.T. was funded by EU TMR Program Research Network contract FMRX-CT98-0164. L.J.M. was supported by postdoctoral fellowship SFRH/BPD/3582/2000 from Fundação para a Ciência e Tecnologia (Portugal), and P.T. is the recipient of funds from the Fonds pour la formation de la Recherche Scientifique dans l'industrie et dans l'agriculture (Belgium). This work was supported by the Belgian Fonds National de la Recherche Scientifique Médicale (convention 3.4595.97) and by the Direction générale de la Recherche Scientifique-Communauté Française de Belgique (Action de Recherche Concertée 94/99-172).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285-294. [DOI] [PubMed] [Google Scholar]
- 2.Bach, S., C. Buchrieser, M. Prentice, A. Guiyoule, T. Msadek, and E. Carniel. 1999. The high-pathogenicity island of Yersinia enterocolitica Ye8081 undergoes low-frequency deletion but not precise excision, suggesting recent stabilization in the genome. Infect. Immun. 67:5091-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuscher, H. U., F. Rodel, A. Forsberg, and M. Rollinghoff. 1995. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect. Immun. 63:1270-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliska, J. B. 2000. Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol. 8:205-208. [DOI] [PubMed] [Google Scholar]
- 5.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd, A. P., N. Grosdent, S. Totemeyer, C. Geuijen, S. Bleves, M. Iriarte, I. Lambermont, J. N. Octave, and G. R. Cornelis. 2000. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79:659-671. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis, G. R. 2000. Type III secretion: a bacterial device for close combat with cells of their eukaryotic host. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:681-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis, G. R., and G. Denecker. 2001. Yersinia lead SUMO attack. Nat. Med. 7:21-23. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 12.Darwin, A. J., and V. L. Miller. 1999. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol. Microbiol. 32:51-62. [DOI] [PubMed] [Google Scholar]
- 13.Delor, I., A. Kaeckenbeeck, G. Wauters, and G. R. Cornelis. 1990. Nucleotide sequence of yst, the Yersinia enterocolitica gene encoding the heat-stable enterotoxin, and prevalence of the gene among pathogenic and nonpathogenic yersiniae. Infect. Immun. 58:2983-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 15.Foultier, B., P. Troisfontaines, S. Müller, F. Opperdoes, and G. R. Cornelis. Characterization of the ysa pathogenicity locus in the chromosome of Yersinia enterocolitica and phylogenic analysis of type III secretion systems. J. Mol. Evol., in press. [DOI] [PubMed]
- 16.Gaede, K. I., and J. Heesemann. 1995. Arthritogenicity of genetically manipulated Yersinia enterocolitica serotype O8 for Lewis rats. Infect. Immun 63:714-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galyov, E. E., S. Hakansson, and H. Wolf-Watz. 1994. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J. Bacteriol. 176:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grutzkau, A., C. Hanski, H. Hahn, and E. O. Riecken. 1990. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 31:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:1436-1446. [DOI] [PubMed] [Google Scholar]
- 20.Heesemann, J., B. Algermissen, and R. Laufs. 1984. Genetically manipulated virulence of Yersinia enterocolitica. Infect. Immun. 46:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichinohe, H., M. Yoshioka, H. Fukushima, S. Kaneko, and T. Maruyama. 1991. First isolation of Yersinia enterocolitica serotype O:8 in Japan. J. Clin. Microbiol. 29:846-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani, A., F. Bussolino, and M. Introna. 1997. Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol. Today 18:231-240. [DOI] [PubMed] [Google Scholar]
- 25.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 32.Orth, K., Z. Xu, M. B. Mudgett, Z. Q. Bao, L. E. Palmer, J. B. Bliska, W. F. Mangel, B. Staskawicz, and J. E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290:1594-1597. [DOI] [PubMed] [Google Scholar]
- 33.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 34.Palmer, L. E., A. R. Pancetti, S. Greenberg, and J. B. Bliska. 1999. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect. Immun. 67:708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portnoy, D. A., S. L. Moseley, and S. Falkow. 1981. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect. Immun. 31:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakin, A., C. Noelting, S. Schubert, and J. Heesemann. 1999. Common and specific characteristics of the high-pathogenicity island of Yersinia enterocolitica. Infect. Immun. 67:5265-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruckdeschel, K., O. Mannel, K. Richter, C. Jacobi, K. Trülzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-κB pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 39.Ruckdeschel, K., K. Richter, O. Mannel, and J. Heesemann. 2001. Arginine-143 of Yersinia enterocolitica YopP crucially determines isotype-related NF-kappaB suppression and apoptosis induction in macrophages. Infect. Immun. 69:7652-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Sarker, M. R., C. Neyt, I. Stainier, and G. R. Cornelis. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schesser, K., A. K. Spiik, J. M. Dukuzumuremyi, M. F. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067-1079. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, A., M. Rollinghoff, and H. U. Beuscher. 1999. Suppression of TNF by V antigen of Yersinia spp. involves activated T cells. Eur. J. Immunol. 29:1149-1157. [DOI] [PubMed] [Google Scholar]
- 44.Schulte, R., P. Wattiau, E. L. Hartland, R. M. Robins-Browne, and G. R. Cornelis. 1996. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect. Immun. 64:2106-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 46.Skrzypek, E., C. Cowan, and S. C. Straley. 1998. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30:1051-1065. [DOI] [PubMed] [Google Scholar]
- 47.Sory, M. P., A. Boland, I. Lambermont, and G. R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA 92:11998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sory, M. P., and G. Cornelis. 1988. Yersinia enterocolitica O:9 as a potential live oral carrier for protective antigens. Microb. Pathog. 4:431-442. [DOI] [PubMed] [Google Scholar]
- 49.Straley, S. C., and W. S. Bowmer. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]