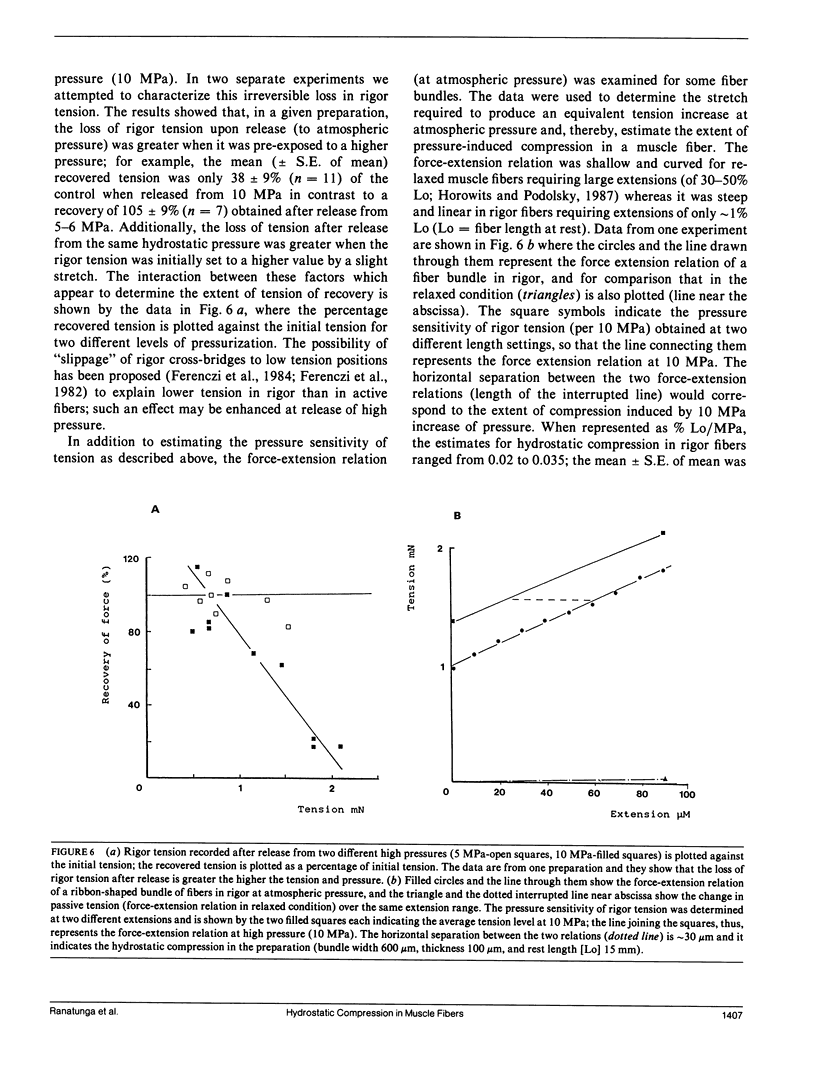
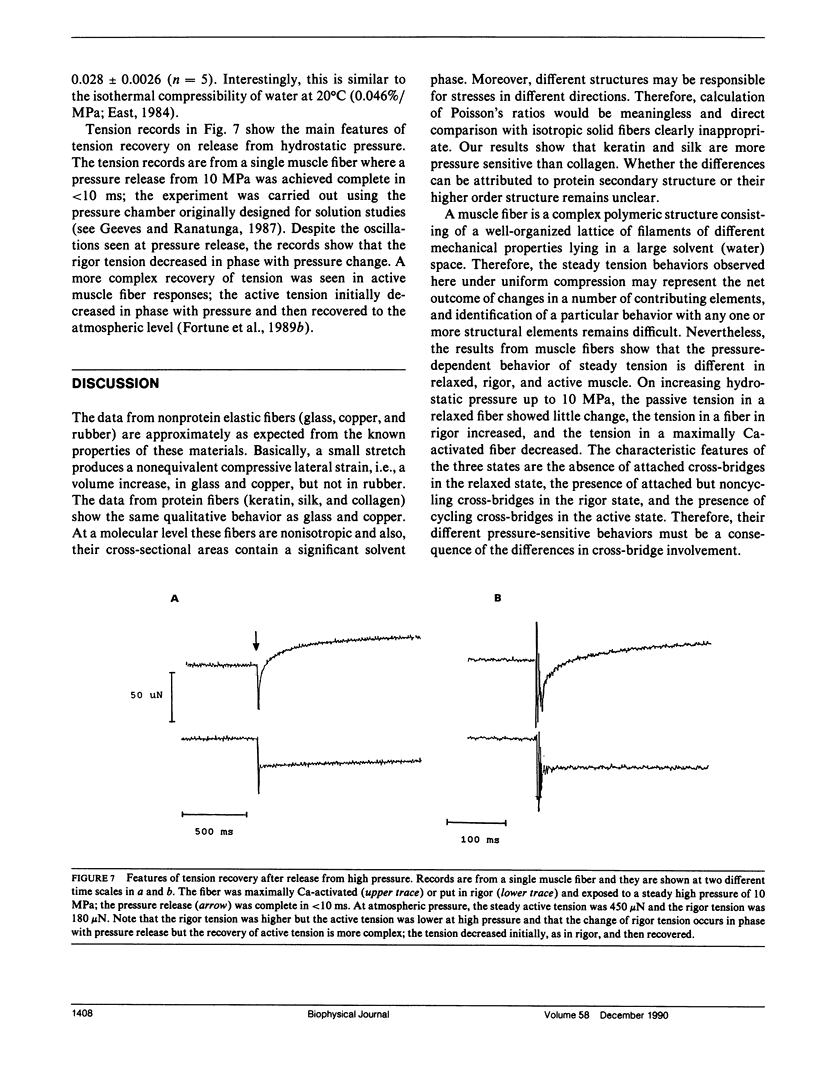
Abstract
Glycerinated muscle fibers isolated from rabbit psoas muscle, and a number of other nonmuscle elastic fibers including glass, rubber, and collagen, were exposed to hydrostatic pressures of up to 10 MPa (100 Atm) to determine the pressure sensitivity of their isometric tension. The isometric tension of muscle fibers in the relaxed state (passive tension) was insensitive to increased pressure, whereas the muscle fiber tension in rigor state increased linearly with pressure. The tension of all other fiber types (except rubber) also increased with pressure; the rubber tension was pressure insensitive. The pressure sensitivity of rigor tension was 2.3 kN/m2/MPa and, in comparison with force/extension relation determined at atmospheric pressure, the hydrostatic compression in rigor muscle fibers was estimated to be 0.03% Lo/MPa. As reported previously, the active muscle fiber tension is depressed by increased pressure. The possible underlying basis of the different pressure-dependent tension behavior in relaxed, rigor, and active muscle is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Simmons R. M., Sleep J. A. General considerations of cross-bridge models in relation to the dependence on MgATP concentration of mechanical parameters of skinned fibers from frog muscles. Soc Gen Physiol Ser. 1982;37:91–107. [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune N. S., Geeves M. A., Ranatunga K. W. Pressure sensitivity of active tension in glycerinated rabbit psoas muscle fibres: effects of ADP and phosphate. J Muscle Res Cell Motil. 1989 Apr;10(2):113–123. doi: 10.1007/BF01739967. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Ranatunga K. W. Tension responses to increased hydrostatic pressure in glycerinated rabbit psoas muscle fibres. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):217–226. doi: 10.1098/rspb.1987.0070. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., McCray J. A., Ranatunga K. W. Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J Physiol. 1987 Nov;392:71–95. doi: 10.1113/jphysiol.1987.sp016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Critical dependence of calcium-activated force on width in highly compressed skinned fibers of the frog. Biophys J. 1985 Nov;48(5):781–787. doi: 10.1016/S0006-3495(85)83836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Kinetics of force redevelopment in isolated intact frog fibers in solutions of varied osmolarity. Biophys J. 1986 Apr;49(4):949–955. doi: 10.1016/S0006-3495(86)83723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Tonicity effects on intact single muscle fibers: relation between force and cell volume. Science. 1982 Feb 26;215(4536):1109–1112. doi: 10.1126/science.6977845. [DOI] [PubMed] [Google Scholar]
- Horowits R., Podolsky R. J. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol. 1987 Nov;105(5):2217–2223. doi: 10.1083/jcb.105.5.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The crossbridge mechanism of muscular contraction and its implications. J Exp Biol. 1985 Mar;115:17–30. doi: 10.1242/jeb.115.1.17. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Matsubara S., Natori R., Nonomura Y., Kimura S. Connectin, an elastic protein of muscle. Characterization and Function. J Biochem. 1977 Aug;82(2):317–337. [PubMed] [Google Scholar]
- Maruyama K., Matsuno A., Higuchi H., Shimaoka S., Kimura S., Shimizu T. Behaviour of connectin (titin) and nebulin in skinned muscle fibres released after extreme stretch as revealed by immunoelectron microscopy. J Muscle Res Cell Motil. 1989 Oct;10(5):350–359. doi: 10.1007/BF01758431. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Shortening velocity in skinned single muscle fibers. Influence of filament lattice spacing. Biophys J. 1987 Jul;52(1):127–131. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Naylor G. R., Arata T. Cross-bridge properties in the rigor state. Soc Gen Physiol Ser. 1982;37:79–89. [PubMed] [Google Scholar]
- Trinick J., Knight P., Whiting A. Purification and properties of native titin. J Mol Biol. 1984 Dec 5;180(2):331–356. doi: 10.1016/s0022-2836(84)80007-8. [DOI] [PubMed] [Google Scholar]
- Wang K., Ramirez-Mitchell R. A network of transverse and longitudinal intermediate filaments is associated with sarcomeres of adult vertebrate skeletal muscle. J Cell Biol. 1983 Feb;96(2):562–570. doi: 10.1083/jcb.96.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ramirez-Mitchell R., Palter D. Titin is an extraordinarily long, flexible, and slender myofibrillar protein. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3685–3689. doi: 10.1073/pnas.81.12.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]


