Abstract
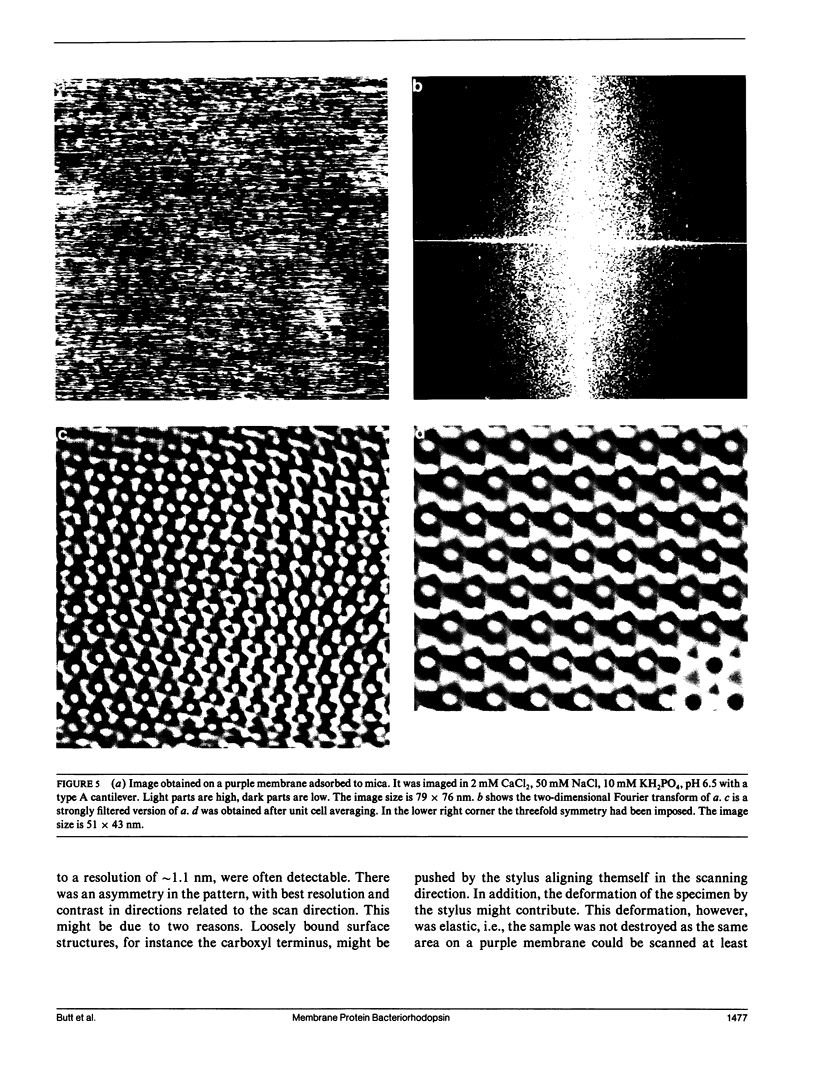
The membrane protein bacteriorhodopsin was imaged in buffer solution at room temperature with the atomic force microscope. Three different substrates were used: mica, silanized glass and lipid bilayers. Single bacteriorhodopsin molecules could be imaged in purple membranes adsorbed to mica. A depression was observed between the bacteriorhodopsin molecules. The two dimensional Fourier transform showed the hexagonal lattice with a lattice constant of 6.21 +/- 0.20 nm which is in agreement with results of electron diffraction experiments. Spots at a resolution of approximately 1.1 nm could be resolved. A protein, cationic ferritin, could be imaged bound to the purple membranes on glass which was silanized with aminopropyltriethoxysilane. This opens the possibility of studying receptor/ligand binding under native conditions. In addition, purple membranes bound to a lipid bilayer were imaged. These images may help in interpreting results of functional studies done with purple membranes adsorbed to black lipid membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Drake B., Prater C. B., Weisenhorn A. L., Gould S. A., Albrecht T. R., Quate C. F., Cannell D. S., Hansma H. G., Hansma P. K. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science. 1989 Mar 24;243(4898):1586–1589. doi: 10.1126/science.2928794. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Zaccai G. Bacteriorhodopsin is an inside-out protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5894–5898. doi: 10.1073/pnas.77.10.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. A., Yanagimoto K., Stoeckenius W. Oriented adsorption of purple membrane to cationic surfaces. J Cell Biol. 1978 May;77(2):611–621. doi: 10.1083/jcb.77.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. Protocol for 3-D visualization of molecules on mica via the quick-freeze, deep-etch technique. J Electron Microsc Tech. 1989 Nov;13(3):244–263. doi: 10.1002/jemt.1060130310. [DOI] [PubMed] [Google Scholar]
- Holz M., Lindau M., Heyn M. P. Distributed kinetics of the charge movements in bacteriorhodopsin: evidence for conformational substates. Biophys J. 1988 Apr;53(4):623–633. doi: 10.1016/S0006-3495(88)83141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe J. S., Glaeser R. M. Difference Fourier analysis of "surface features" of bacteriorhodopsin using glucose-embedded and frozen-hydrated purple membrane. Ultramicroscopy. 1987;23(1):17–28. doi: 10.1016/0304-3991(87)90223-3. [DOI] [PubMed] [Google Scholar]
- Jubb J. S., Worcester D. L., Crespi H. L., Zaccaï G. Retinal location in purple membrane of Halobacterium halobium: a neutron diffraction study of membranes labelled in vivo with deuterated retinal. EMBO J. 1984 Jul;3(7):1455–1461. doi: 10.1002/j.1460-2075.1984.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti O., Ribi H. O., Drake B., Albrecht T. R., Quate C. F., Hansma P. K. Atomic force microscopy of an organic monolayer. Science. 1988 Jan 1;239(4835):50–52. doi: 10.1126/science.3336773. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Engelman D. M., Gurel O., Zaccaï G. Tertiary structure of bacteriorhodopsin. Positions and orientations of helices A and B in the structural map determined by neutron diffraction. J Mol Biol. 1989 Dec 20;210(4):829–847. doi: 10.1016/0022-2836(89)90111-3. [DOI] [PubMed] [Google Scholar]
- Seiff F., Westerhausen J., Wallat I., Heyn M. P. Location of the cyclohexene ring of the chromophore of bacteriorhodopsin by neutron diffraction with selectively deuterated retinal. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7746–7750. doi: 10.1073/pnas.83.20.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Trewhella J., Anderson S., Fox R., Gogol E., Khan S., Engelman D., Zaccai G. Assignment of segments of the bacteriorhodopsin sequence to positions in the structural map. Biophys J. 1983 Jun;42(3):233–241. doi: 10.1016/S0006-3495(83)84391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcester D. L., Miller R. G., Bryant P. J. Atomic force microscopy of purple membranes. J Microsc. 1988 Dec;152(Pt 3):817–821. doi: 10.1111/j.1365-2818.1988.tb01454.x. [DOI] [PubMed] [Google Scholar]