Abstract
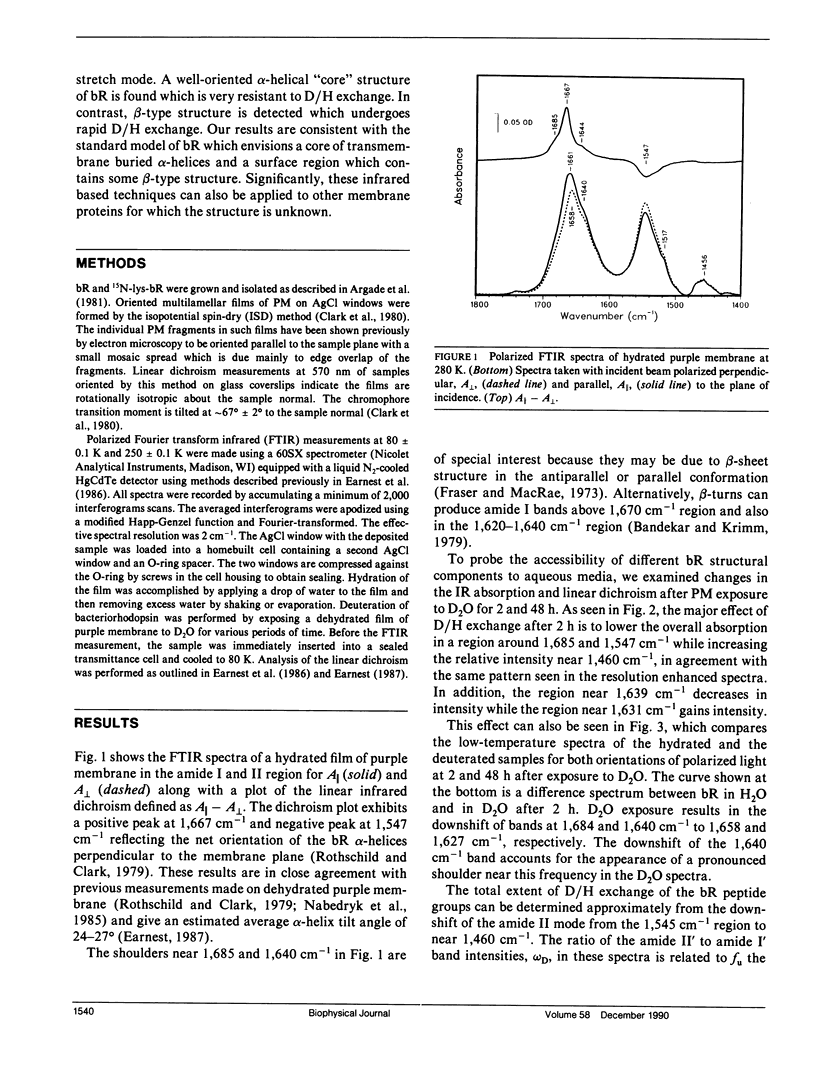
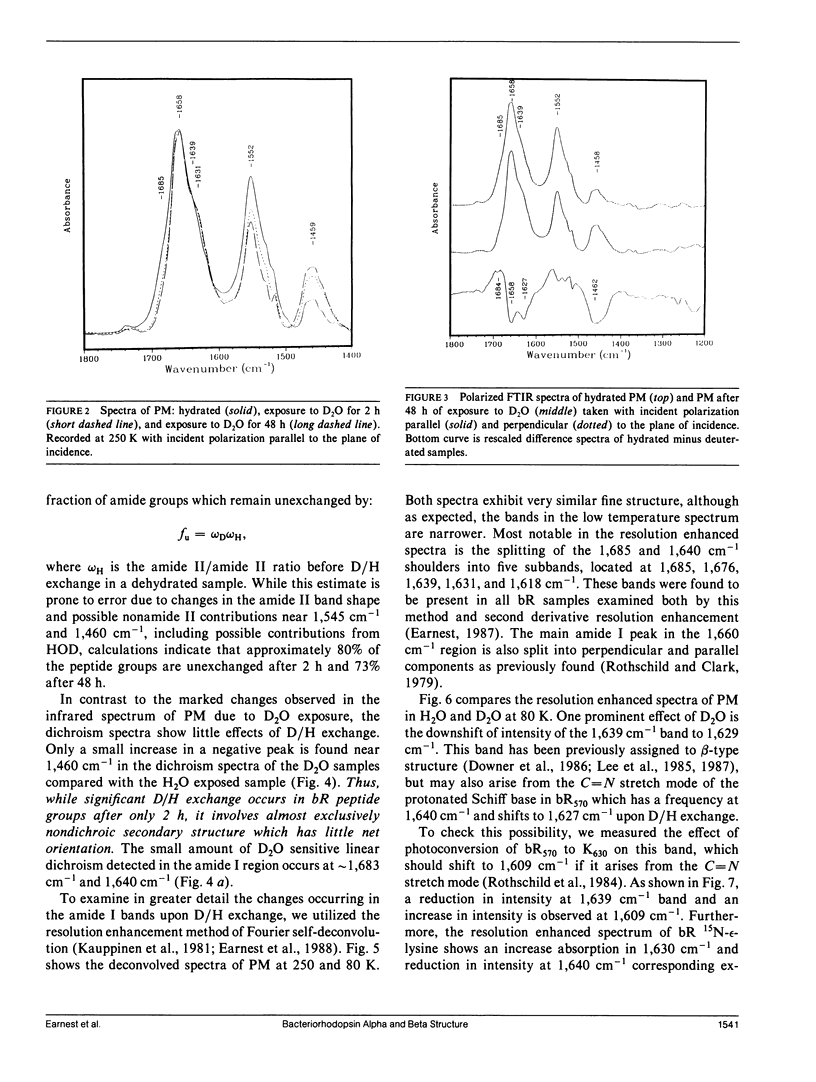
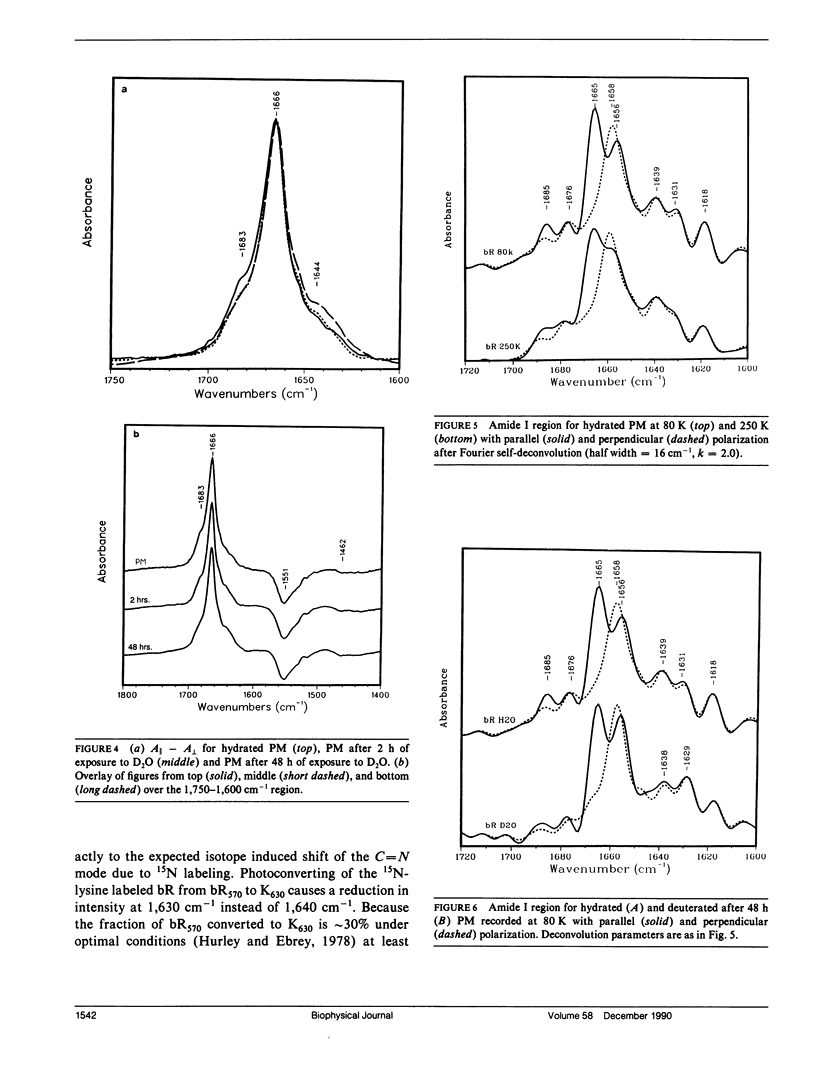
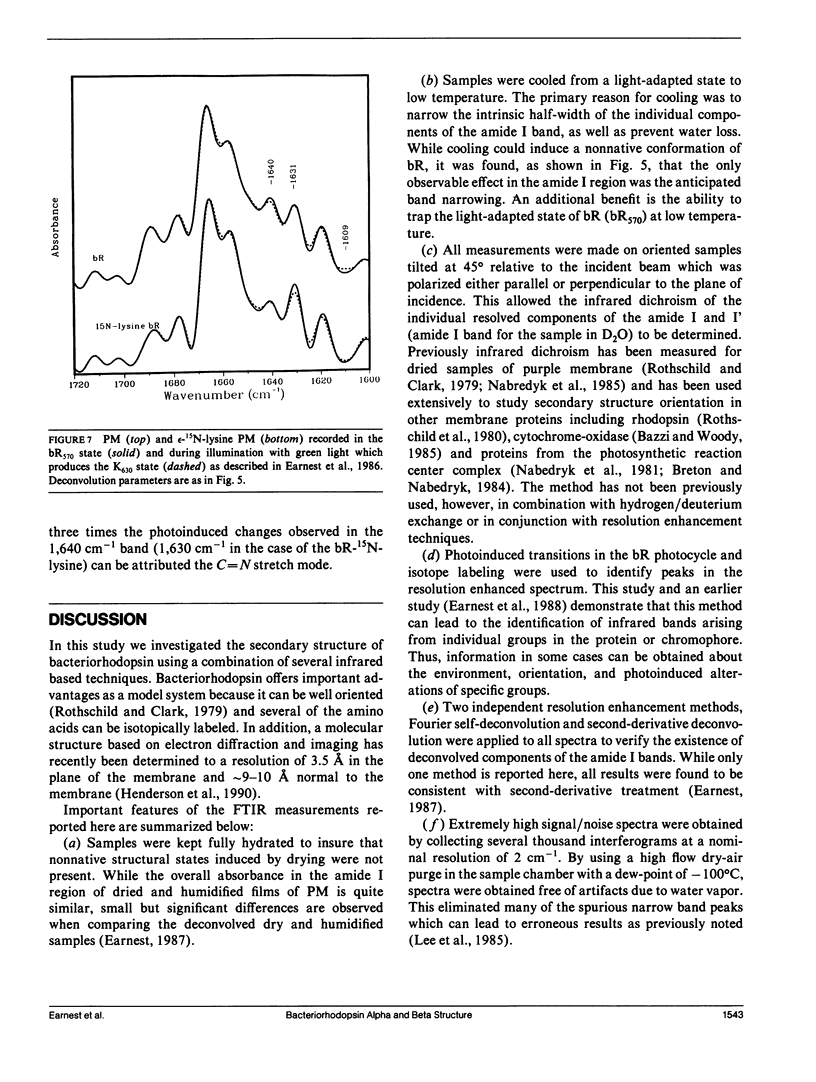
The secondary structure of bacteriorhodopsin has been investigated by polarized Fourier transform infrared spectroscopy combined with hydrogen/deuterium exchange, isotope labeling and resolution enhancement methods. Oriented films of purple membrane were measured at low temperature after exposure to H2O or D2O. Resolution enhancement techniques and isotopic labeling of the Schiff base were used to assign peaks in the amide I region of the spectrum. alpha-helical structure, which exhibits strong infrared dichroism, undergoes little H/D exchange, even after 48 h of D2O exposure. In contrast, non-alpha-helical structure, which exhibits little dichroism, undergoes rapid H/D exchange. A band at 1,640 cm-1, which has previously been assigned to beta-sheet structure, is found to be due in part to the C = N stretching vibration of protonated Schiff base of the retinylidene chromophore. We conclude that the membrane spanning regions of bR consist predominantly of alpha-helical structure whereas most beta-type structure is located in surface regions directly accessible to water.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahl P. L., Stern L. J., Düring D., Mogi T., Khorana H. G., Rothschild K. J. Effects of amino acid substitutions in the F helix of bacteriorhodopsin. Low temperature ultraviolet/visible difference spectroscopy. J Biol Chem. 1988 Sep 25;263(27):13594–13601. [PubMed] [Google Scholar]
- Argade P. V., Rothschild K. J., Kawamoto A. H., Herzfeld J., Herlihy W. C. Resonance Raman spectroscopy of specifically [epsilon-15N]lysine-labeled bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1643–1646. doi: 10.1073/pnas.78.3.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandekar J., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins: Characteristic amide bands of beta-turns. Proc Natl Acad Sci U S A. 1979 Feb;76(2):774–777. doi: 10.1073/pnas.76.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi M. D., Woody R. W. Oriented secondary structure in integral membrane proteins. I. Circular dichroism and infrared spectroscopy of cytochrome oxidase in multilamellar films. Biophys J. 1985 Dec;48(6):957–966. doi: 10.1016/S0006-3495(85)83859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Bruchman T. J., Hazzard J. H. Infrared spectroscopic study of photoreceptor membrane and purple membrane. Protein secondary structure and hydrogen deuterium exchange. J Biol Chem. 1986 Mar 15;261(8):3640–3647. [PubMed] [Google Scholar]
- Earnest T. N., Roepe P., Braiman M. S., Gillespie J., Rothschild K. J. Orientation of the bacteriorhodopsin chromophore probed by polarized Fourier transform infrared difference spectroscopy. Biochemistry. 1986 Dec 2;25(24):7793–7798. doi: 10.1021/bi00372a002. [DOI] [PubMed] [Google Scholar]
- Englander J. J., Englander S. W. Comparison of bacterial and animal rhodopsins by hydrogen exchange studies. Nature. 1977 Feb 17;265(5595):658–659. doi: 10.1038/265658a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G. Energy transfer in the purple membrane of Halobacterium halobium. Biophys J. 1978 Apr;22(1):49–66. doi: 10.1016/S0006-3495(78)85470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jap B. K., Maestre M. F., Hayward S. B., Glaeser R. M. Peptide-chain secondary structure of bacteriorhodopsin. Biophys J. 1983 Jul;43(1):81–89. doi: 10.1016/S0006-3495(83)84326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm S., Dwivedi A. M. Infrared spectrum of the purple membrane: clue to a proton conduction mechanism? Science. 1982 Apr 23;216(4544):407–408. doi: 10.1126/science.6280277. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Hayward J. A., Restall C. J., Chapman D. Second-derivative infrared spectroscopic studies of the secondary structures of bacteriorhodopsin and Ca2+-ATPase. Biochemistry. 1985 Jul 30;24(16):4364–4373. doi: 10.1021/bi00337a018. [DOI] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Chao B. H., Khorana H. G. Structure-function studies on bacteriorhodopsin. VIII. Substitutions of the membrane-embedded prolines 50, 91, and 186: the effects are determined by the substituting amino acids. J Biol Chem. 1989 Aug 25;264(24):14192–14196. [PubMed] [Google Scholar]
- Nabedryk E., Bardin A. M., Breton J. Further characterization of protein secondary structures in purple membrane by circular dichroism and polarized infrared spectroscopies. Biophys J. 1985 Dec;48(6):873–876. doi: 10.1016/S0006-3495(85)83848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J. Fourier transform infrared studies of an active proton transport pump. Methods Enzymol. 1986;127:343–353. doi: 10.1016/0076-6879(86)27028-7. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., He Y. W., Gray D., Roepe P. D., Pelletier S. L., Brown R. S., Herzfeld J. Fourier transform infrared evidence for proline structural changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9832–9835. doi: 10.1073/pnas.86.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Marrero H., Braiman M., Mathies R. Primary photochemistry of bacteriorhodopsin: comparison of Fourier transform infrared difference spectra with resonance Raman spectra. Photochem Photobiol. 1984 Nov;40(5):675–679. doi: 10.1111/j.1751-1097.1984.tb05359.x. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Sanches R., Hsiao T. L., Clark N. A. A spectroscopic study of rhodopsin alpha-helix orientation. Biophys J. 1980 Jul;31(1):53–64. doi: 10.1016/S0006-3495(80)85040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Vogel H., Gärtner W. The secondary structure of bacteriorhodopsin determined by Raman and circular dichroism spectroscopy. J Biol Chem. 1987 Aug 25;262(24):11464–11469. [PubMed] [Google Scholar]
- Wallace B. A., Teeters C. L. Differential absorption flattening optical effects are significant in the circular dichroism spectra of large membrane fragments. Biochemistry. 1987 Jan 13;26(1):65–70. doi: 10.1021/bi00375a010. [DOI] [PubMed] [Google Scholar]


