Abstract
Members of the variable merozoite surface antigen (vmsa) gene family of Babesia bovis encode membrane proteins involved in erythrocyte invasion. In this study, we have identified and sequenced the complete 8.3-kb genomic locus containing msa-2, a member of the vmsa family, in the biologically cloned Mexico Mo7 strain. Four tandemly arranged copies of msa-2-related genes were found in the locus. The four genes, designated msa-2a1 (which corresponds to the originally described msa-2 gene), msa-2a2, msa-2b, and msa-2c, were shown to be transcribed and expressed and encode proteins with open reading frames ranging in size from 266 (MSA-2c) to 317 (MSA-2a1) amino acids. MSA-2a1 and -2a2 are the most closely related of the four proteins (90% identity), differing by (i) the number of 24-amino-acid repeats that comprise a surface-exposed B-cell epitope and (ii) the presence of a 32-amino-acid area of recombination between MSA-2a2 and -2b. In contrast, msa-2c is most closely related to the previously described babr 0.8 gene in Australia strains of B. bovis. Comparison of MSA-2 proteins in the Argentina R1A strain of B. bovis with the Mexico Mo7 clone revealed a relatively high degree of conservation (83.6, 69.4, 79.1, and 88.7% amino acid identity for MSA-2a1, -2a2, -2b, and -2c, respectively), in contrast to the extensive MSA-1 sequence variation (52% identity) between the same two strains. Postinfection bovine immune serum contains antibodies that bound to each of the recombinant MSA-2 proteins. Blocking assays demonstrated the presence of unique B-cell epitopes in MSA-2a1, -2b, and -2c. The results support the evolution of the msa-2 locus through at least two gene duplications, with selection for multiple related but antigenically distinct merozoite surface proteins.
The Babesia bovis variable merozoite surface antigens (VMSA) are surface-exposed glycoproteins anchored in the cell membrane by glycosyl-phosphatidylinositol (GPI) moieties (10). Members of this gene family include msa-1 and -2, encoding 42- and 44-kDa proteins, respectively, in the biologically cloned Mexico Mo7 strain, and at least three babr genes identified in the Australia K strain (3, 10, 12). Based on their surface location and the ability of antibodies against MSA-1 to block merozoite entry into erythrocytes, members of the VMSA family have been proposed to function in erythrocyte invasion and are considered candidate vaccine antigens (2, 10, 18).
In contrast to proteins that are expressed in the apical complex of the parasite, the VMSA family contains surface-exposed epitopes that are not conserved among geographic strains (19). One polymorphic B-cell epitope has been mapped to a repeat region in the only previously characterized MSA-2 protein (originally termed Bo44) (12, 20). In MSA-1, antigenic polymorphism between strains is the result of distinct alleles encoding markedly variant molecules (26), but the molecular basis for MSA-2 polymorphism has not been characterized. In addition, while the existence of at least five members of the VMSA gene family in the biologically cloned Mo7 strain has been demonstrated (10, 12), analysis of the complete 5.6-kb msa-1 gene locus showed that msa-1 is present as a single copy and that no other vmsa genes occur in the flanking regions (26). Thus, the sequences, pattern of transcription and translation, and immunological features of the remaining members of the vmsa family are unknown.
In this study, we identified and sequenced the locus of msa-2 contained within an 8.3-kb genomic fragment of the Mo7 strain. The locus contains four tandemly arranged msa-2-related genes that appear to have arisen through gene duplication followed by insertion or deletion and intergenic recombination. All four genes are transcribed and expressed in merozoites, and at least three contain unique B-cell epitopes. One of the newly identified msa-2 genes (msa-2c) is most closely related to babr 0.8 in the B. bovis Australia K strain (3). The sequences of all msa-2 genes and proteins are relatively conserved in the Argentina R1A B. bovis strain compared to those of the markedly polymorphic msa-1 alleles (26), suggesting that functional constraints to variation of msa-2 genes may result in conserved targets for protective immunity.
MATERIALS AND METHODS
Parasites.
The B. bovis Mo7 biological clone, derived by limiting dilution of the Mexico strain as described elsewhere (9), and the Argentina R1A strain (1), kindly provided by Ignacio Echaide, were used in this study. Parasites were grown in long-term microaerophilus stationary-phase culture in bovine erythrocytes according to previously described techniques (15).
Isolation and sequencing of the msa-2 locus.
A λ/EMBL-Mo7 BamHI genomic library (28) was used. Phages were plated on Escherichia coli XL1-Blue MRA (P2) cells and screened with the digoxigenin-labeled oligonucleotide probe msa-2-F1 (Table 1), which corresponds to the first 18 nucleotides (nt) of the msa-2 gene as described by Jasmer et al. (12). Screening of this library resulted in the identification of a clone containing an 8.3-kb fragment (λ/EMBL locus), which was subcloned into BamHI-digested plasmid pBluescript (pBS-msa2locus). After sequencing the first 400 bp of each end of the pBS-msa2locus, two primers were designed: PBS44-2F in the 5′ region of the locus and PBS44-2R in the 3′ region of the locus (Table 1). By using these primers and primer msa-2-F1 and its antisense sequence, msa-2-R2 (Table 1), two subfragments were generated by PCR with Taq DNA polymerase (Gibco Life Technologies) and B. bovis Mo7 DNA as a template and cloned into vector pCR 2.1 for sequencing: (i) msa2-sub1 (approximately 3.0 kb) with primers PBS44-2F and msa-2-R2; and (ii) msa2-sub2 (approximately 1.5 kb) with primers PBS44-2R and msa-2-F1. msa2-sub1, which corresponds to the 5′ region of the msa-2 locus, contained a previously unknown msa-2 gene, here denominated msa-2c, and the 5′ region of the previously described msa-2 gene, which was temporarily designated msa-2a. Subclone msa2-sub2 contained another previously undescribed msa-2 gene, here denominated msa-2b. Following sequencing of msa2-sub2, the remaining approximately 4.0-kb fragment was amplified from genomic Mo7 DNA with primers msa-2-F1 and msa-2b-R (Table 1). This subfragment (msa2-sub3) was also cloned into vector pCR 2.1 and sequenced. It contained msa-2a and another very similar msa-2 gene. These genes were therefore designated msa-2a1 and msa-2a2, respectively. A shorter amplification product with primers msa-2-F1 and msa-2b-R also was cloned into pCR 2.1, and its sequence was used to confirm that of msa2-sub3. The location of the three subfragments in the msa-2 locus is shown in Fig. 1B. Sequences of the open reading frames (ORFs) of all four msa-2 genes in the locus were confirmed by sequencing two additional independent PCR-generated clones for each gene: one from plasmid pBS-msa2locus and one from cDNA after reverse transcription. Sequences from all three clones were identical for each msa-2 gene. In all cases, sequencing was carried out with the ABI PRISM BigDye Terminator cycle sequencing V2.0 ready reaction kit. The samples were analyzed on an ABI Prism 377 or ABI Prism 373 automated DNA sequencer (Applied Biosystems). The resulting sequences were assembled and analyzed with the University of Wisconsin Genetics Computer Group program (version 10.0). Sequence comparisons, structural analysis, and predicted antigenicity were carried out with Vector Suite NTI Suite 6 (InforMax), and the Baylor College of Medicine search launcher (http://searchlauncher.bcm.tmc.edu) (11). Phylogenetic analysis was performed with PHYLIP (Phylogeny Inference Package) version 3.57c (4). Following ClustalW alignment, bootstrapping (100 replicates) of the sequence data set was conducted with the program SEQBOOT with B. bovis rap1 as an outlier, and the best tree was determined by the parsimony method for DNA (DNApars) and CONSENSE analysis.
TABLE 1.
Oligonucleotides used for sequencing and amplification of msa-2 genes
| Primer | Application | Sequence |
|---|---|---|
| msa-2-F1 | Sequencing, PCR | 5′-ATGATCGGGAAAATCTTC-3′ |
| msa-2-R2 | Sequencing, PCR | 5′-GAAGATTTTCCCGATCAT-3′ |
| PBS44-2F | Sequencing, PCR | 5′-GGTAATGGAATTTATGTGCAAC-3′ |
| PBS44-2R | Sequencing, PCR | 5′-CAATATGTACCTTGGCTGATG-3′ |
| B42/44-R | PCR, RT-PCR | 5′-AAAATGCAGAGAGAACG-3′ |
| msa-2a1-F | RT-PCR | 5′-GACGAATGGGAAAGAAAAAATG-3′ |
| msa-2a1-R | RT-PCR | 5′-GCTAGGTGCAGCTGAATCC-3′ |
| msa-2a2-F | RT-PCR | 5′-GGCGGATAAAAACGGAGAAGTG-3′ |
| msa-2a2-R | RT-PCR | 5′-GAGGAGAGGGAGCTGC-3′ |
| msa-2b-F | RT-PCR | 5′-GAAAGAAGAAGGTGGACATTATT-3′ |
| msa-2b-R | Sequencing, RT-PCR | 5′-GTCCACCTGCAGATTCAGATG-3′ |
| msa-2c-F | PCR, RT-PCR | 5′-ATGGTGTCTTTTAACATAATAAC-3′ |
FIG. 1.
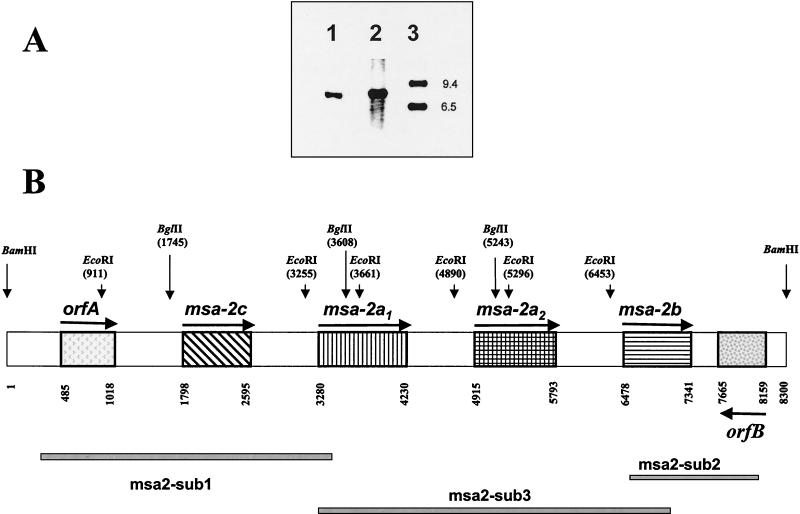
The B. bovis Mo7 msa-2 locus has four tandemly arranged msa-2 genes. (A) Southern blot of BamHI-treated genomic DNA from B. bovis Mo7 (lane 1) and DNA from a λ/EMBL-phage clone (λ/EMBL locus) containing an 8.3-kb genomic fragment (lane 2) with the msa-2-specific digoxigenin-labeled oligonucleotide msa-2-F1 (Table 1). Lane 3, DNA ladder. Hybridization was detected by chemiluminescence. (B) Map of the Mo7 msa-2 locus based on sequencing of the 8.3-kb genomic fragment. ORFs are indicated by patterned boxes. Arrows indicate the orientation of the coding DNA strand. orfA shows no significant homology to other known genes, and orfB has strong homology to several thiolases and acetyl-CoA acetyltransferases. Restriction sites for BamHI, HindIII, BglII, and EcoRI are indicated. The subfragments msa2-sub1, msa2-sub2, and msa2-sub3 shown at the bottom were generated by PCR from Mo7 DNA for sequencing.
Amplification of msa-2 genes from B. bovis R1A strain.
Genomic DNA extracted from B. bovis R1A cultures was amplified by Taq polymerase (Gibco Life Technologies) with primer set msa-2-F1 and B42/44-R for msa-2a1, -2a2, and -2b, and primer set msa-2c-F and B42/44-R for msa-2c (Table 1). The amplification products were cloned into vector pCR 2.1. Twenty clones were analyzed for each transformation. Three types of clones were produced with the first set of primers and sequence analysis showed that they corresponded to the R1A orthologues of Mo7 msa-2a1, -2a2, and -2b. All clones amplified with the second set of primers contained an insert corresponding to the R1A orthologue of msa-2c.
Transcriptional analysis.
mRNA was extracted from purified B. bovis strain Mo7 merozoite cultures by using oligo(dT) affinity columns following the manufacturer's instructions (Ambion, Inc.) and converted to cDNA by using the SuperScript II RNase H reverse transcriptase (RT) first-strand synthesis system (Gibco BRL). A negative control containing mRNA and no RT was produced in parallel. Selective PCR amplification of each msa-2 gene was carried out with cDNA, no-RT mRNA, and genomic DNA by denaturing at 94°C for 2 min, followed by 35 cycles of 30 s at 94°C, 30 s at 62°C (msa-2a1, -2a2, and -2b) or 58°C (for msa-2c), and 1 min at 72°C. The primers used are listed in Table 1 and were as follows: msa-2a1-F and msa-2a1-R for msa-2a1, msa-2a2-F and msa-2a2-R for msa-2a2, msa-2b-F and msa-2b-R for msa-2b, and msa-2c-F and B42/44R for msa-2c. The specificity of the primer pairs for each gene was confirmed with recombinant constructs as a template, and amplicons produced from cDNA and DNA were cloned into vector pCR 2.1 (Invitrogen) and sequenced.
Expression analysis. (i) Recombinant MSA-2 proteins.
Recombinant proteins of the four msa-2 genes were obtained by using the prokaryotic expression system pBAD/thio-TOPO (Invitrogen). The four genes were amplified from B. bovis Mo7 DNA with primers msa-2-F1 and B42/44R for msa-2a1, -2a2, and -2b and msa2c-F and B42/44R for msa-2c. These primers were selected to allow in-frame cloning of the amplicons into the pBAD/thio-TOPO vector. Clones were sequenced to confirm their identity and the correct orientation of the inserts. One clone was selected for each gene, and expression of thioredoxin fusion proteins was effected in early-logarithmic-phase E. coli-transformed cultures (125 ml) after induction with 0.2% arabinose for 3 h at 37°C. Bacterial pellets were collected by centrifugation, resuspended in 5 ml of PNLB (50 mM K2HPO4, 400 mM NaCl, 100 mM KCl, 10% glycerol, 0.5% Triton X-100, 10 mM imidazole [pH 7.8]), sonicated, and centrifuged (27,000 × g, 15 min). The resulting pellets were resuspended in 5 ml of 6 M guanidine-HCl-100 mM K2HPO4-10 mM Tris-HCl (pH 8.0) (guanidine buffer) and sonicated to solubilize the bacterial inclusion bodies containing the recombinant proteins. After centrifugation (27,000 × g, 15 min), the supernatants were chromatographed on 1-ml nickel-agarose columns (Invitrogen) equilibrated with guanidine buffer. Columns were washed successively with 10 ml of guanidine buffer; 5 ml of 8 M urea-100 mM K2HPO4 (pH 8.0), 5 ml of 8 M urea-100 mM K2HPO4-10 mM Tris (pH 6.3), and 10 ml of PNB (same composition as PNLB, but without addition of Triton X-100). Elution was effected with 3-ml portions of increasing concentrations of imidazole (0.1 to 0.5 M in PNB) and assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis after staining with Coomassie blue. Proteins were thoroughly dialyzed against phosphate-buffered saline (PBS) and quantitated with the Pierce Micro BCA (bicinchoninic acid) protein assay reagent kit (Pierce).
(ii) Monospecific MSA-2 antisera.
Polyclonal antibodies against MSA-2a1 and -2b were obtained by immunization of mice with 10 μg of purified recombinant protein emulsified in complete Freund's adjuvant, followed by three immunizations each with 10 μg of recombinant protein in incomplete Freund's adjuvant. Murine antisera were analyzed for reactivity and specificity in immunoblots against recombinant MSA-2 antigens and B. bovis Mo7-infected erythrocytes as previously described (27).
Identification of unique epitopes in MSA-2 proteins.
Bovine preimmune serum and serum 2 weeks postchallenge from an animal infected with the T2Bo strain (6, 19) were diluted 1:500 in blocking solution (PBS, 0.2% blocking reagent [Tropix], 0.5% Tween 20) and adsorbed with a lysate of E. coli cells expressing an unrelated recombinant protein, Anaplasma marginale MSP-5 (29) (48-μg/ml final concentration). Cloning and expression of msp-5 were conducted as described above for the msa-2 genes. After a 3-h, 0.2% arabinose induction of a 125-ml E. coli culture transformed with pBAD/thio-TOPO vector containing in-frame msp-5, the cells were pelleted, resuspended in 5 ml of PBS-0.5% Tween 20, and sonicated. Diluted sera were incubated with an aliquot of this lysate for 60 min at 37°C and centrifuged (27,000 × g, 15 min), and the supernatants were collected. Antibody reactivity in E. coli-adsorbed postinfection immune serum to specific MSA-2 proteins was blocked by addition of purified recombinant MSA-2a1, -2a2, -2b, or -2c (final concentration, 8 μg/ml). Preinfection and immune sera, with and without addition of recombinant proteins for blocking, were analyzed in immunoblots with purified recombinant MSA-2 proteins as antigens.
Nucleotide sequence accession number.
The nucleotide sequences presented herein are available in GenBank under the following accession numbers: Mo7 msa-2 locus, AY052538; R1A msa-2a1, AYAY052539; R1A msa-2a2, AY052540; R1A msa-2b, AY052541; and R1A msa-2c, AY052542. Protein identification numbers for ORFs presented herein are as follows: Mo7 orfA, AAL15419; Mo7 MSA-2c, AAL 15420; Mo7 MSA-2a1, AAL15421; Mo7 MSA-2a2, AAL15422; Mo7 MSA-2b, AAL15423; Mo7 orfB, AAL15424; R1A MSA-2a1, AAL15425; R1A MSA-2a2, AAL15426; R1A MSA-2b, AAL15427; and R1A MSA-2c, AAL15428.
RESULTS
Structure of the msa-2 locus.
A BamHI genomic library in λ-EMBL phage from the biologically cloned B. bovis Mo7 strain was screened by using the digoxigenin-labeled oligonucleotide msa-2-F1 (Table 1), corresponding to the first 18 nt of the 5′ terminus of msa-2. DNA from a hybridizing λ/EMBL phage clone (λ/EMBL locus) was purified, digested with BamHI, and analyzed by Southern blotting, together with genomic BamHI-digested DNA from B. bovis. The results (Fig. 1A) show that msa-2-F1 hybridizes with a single 8.3-kb BamHI fragment both in λ/EMBL locus DNA (lane 2) and in genomic DNA (lane 1), suggesting that the phage clone λ/EMBL locus contains all msa-2 genes that share a 5′ end recognized by this probe. The 8.3-kb genomic fragment was subcloned into pBluescript plasmid (pBS-msa2locus) and sequenced.
The structure of the msa-2 locus derived from sequence analysis of plasmid pBS-msa2locus and PCR-generated subclones msa2-sub1, -sub2, and -sub3 is shown in Fig. 1B. The locus contains four tandemly arranged msa-2-related genes, ranging in size from 795 to 948 bp, which we have denominated (based on their order of discovery and relatedness) msa-2a1, -2a2, -2b, and -2c, arranged in the following 5′-to-3′ order: msa-2c, -2a1, -2a2, and -2b. Of these, msa-2a1 is identical in sequence to the msa-2 gene originally described by Jasmer and coworkers (12) (GenBank accession no. M80467), while the other msa-2 genes were unknown prior to this study. The four msa-2 genes are separated by three identical 0.7-kb intergenic regions. In contrast, the 5′ sequence upstream of msa-2c is different from this common intergenic region, suggesting that transcriptional regulation of msa-2c could be different from that of the other three msa-2 genes.
Separated by 783 bp upstream of msa-2c is a complete ORF of 529 bp (orfA in Fig. 1B) in the same coding orientation as the msa-2 genes. Located 333 bp downstream of msa-2b is another complete ORF of 1,029 bp (orfB in Fig. 1B) in reverse coding orientation relative to the msa-2 genes. Neither orfA nor orfB is related to the vmsa family and thus defines the boundaries of the msa-2 gene locus in this DNA fragment. While orfA has no significant homology to other genes (Entrez Nucleotide Database, October 2001), orfB has strong homology to genes coding for thiolases from Clostridium acetobutylicum (gb|AAC26026.1|) and Pseudomonas aeruginosa (gb|AAB48515.1|), as well as those coding for acetyl coenzyme A (acetyl-CoA) acetyltransferases from several species, including Rickettsia prowazekii (pir| |E71633).
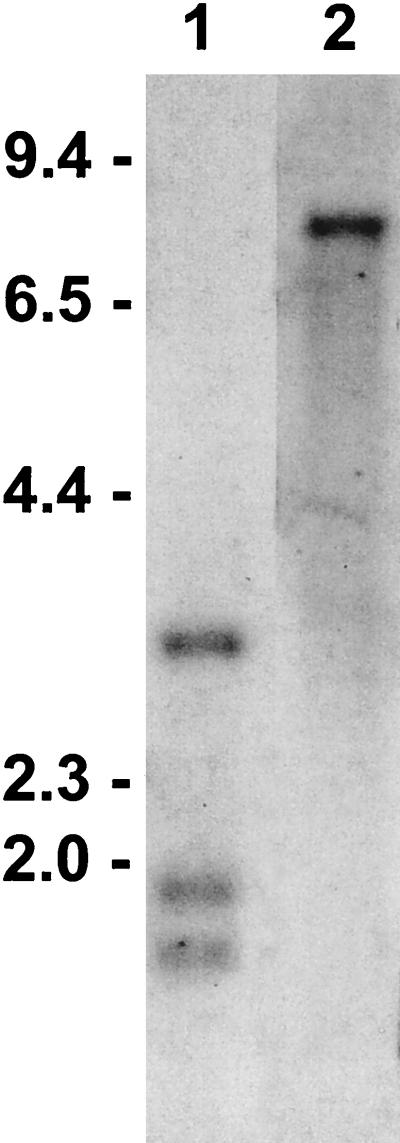
Taking advantage of the sequence differences among the msa-2 genes, we confirmed the gene arrangement in the msa-2 genomic locus shown in Fig. 1B by Southern blot analysis of Mo7 genomic DNA (Fig. 2). Double digestion with BamHI and BglII and probing with digoxigenin-labeled msa-2-F1 identified the expected three bands with approximate sizes of 1.6, 1.8, and 3.1 kb (lane 1), in agreement with the restriction sites of BglII (Fig. 1B). These fragments correspond to the three msa-2 genes (msa-2a1, -2a2, and -2b) that share the msa-2-F1 sequence in their 5′ termini. Since the oligonucleotide probe used in the screening of the BamHI library (msa-2-F1) can hybridize with msa-2a1, -2a2, and -2b, but not with msa-2c, it was necessary to investigate if other msa-2c genes could be detected outside the msa-2 locus. Lane 2 in Fig. 2 shows that BamHI-digested genomic Mo7 DNA probed with digoxigenin-labeled msa-2c-F, which corresponds to the unique first 18 bp of the msa-2c gene (see below), yields a single band of approximately 8.3 kb, indicating that other msa-2c genes outside the msa-2 locus either do not exist or could not be detected under these stringency conditions.
FIG. 2.
Confirmation of gene arrangement within the msa-2 locus. B. bovis Mo7 DNA was treated with BamHI plus BglII (lane 1) or BamHI (lane 2) and analyzed by Southern blotting with digoxigenin-labeled oligonucleotide probes corresponding in lane 1 to the initial 18 5′ nt of msa-2a1, -2a2, and -2b (oligonucleotide msa-2-F1 in Table 1) or in lane 2 to the initial 18 5′ nt of msa-2c (oligonucleotide msa-2c-F in Table 1). Hybridization was detected by chemiluminescence. Size markers in kilobases are shown on the left.
Sequence analysis of msa-2 genes.
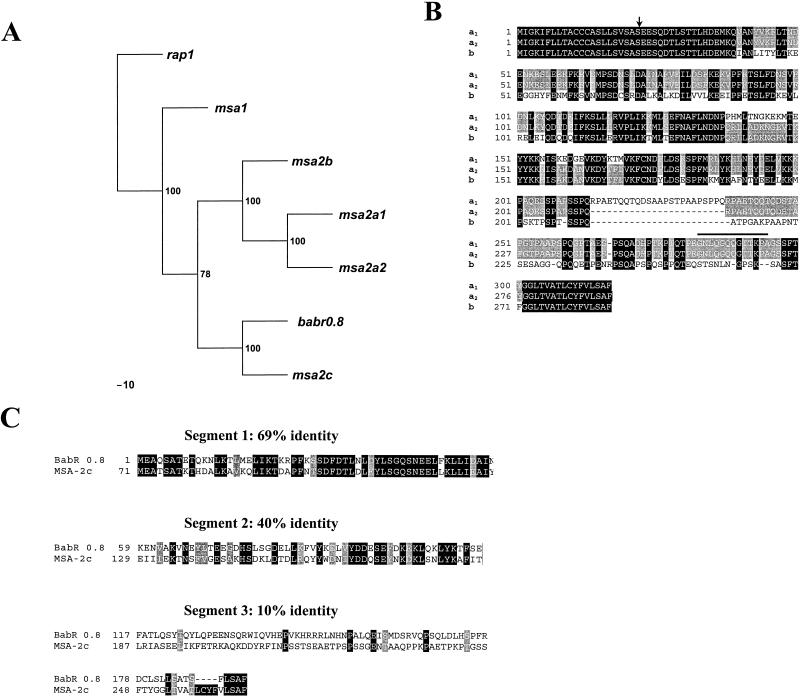
A phylogenetic analysis of the four msa-2 genes in relation to all known genes in the vmsa family is shown in Fig. 3A. The unrelated B. bovis rap-1 gene was used as an outlier. Bootstrap values were determined with the SEQBOOT program of the PHYLIP package (4) with 100 replicates, and the best tree was constructed with DNAPARS and CONSENSE. This analysis indicates that msa-2a1, -2a2, and -2b cluster separately from msa-2c and that msa-2c is most closely related to the babr 0.8 gene originally described in the Australia K strain (3) and was later shown to be a member of the vmsa family (10). It also suggests that msa-1 has a more distant, perhaps ancestral, relationship to other members of the vmsa family.
FIG. 3.
Sequence comparisons of B. bovis Mo7 msa-2 genes and encoded proteins. (A) Phylogram of the vmsa family. B. bovis rap1 is used as an outlier, and the most parsimonious tree is shown. Bootstrap values are indicated at branch points. (B) Alignment of MSA-2a1, -2a2, and -2b. Areas of amino acid identity among all three proteins are enclosed in black boxes; amino acids conserved between two of the proteins have a gray background, and variant amino acids have a white background. Deletions are indicated by dashed lines. The sequence conserved among MSA-1, -2a1, and -2a2 is indicated by a bold line above the sequence, and the predicted site for cleavage of the amino-terminal signal peptide is indicated by an arrow. (C) Fractionated alignment and sequence comparison between Mo7 MSA-2c and Australia K strain BabR 0.8. The amino acid sequences were aligned and divided into three segments according to the degree of conservation. The ORF of BabR 0.8 starts 71 aa downstream from the start codon of MSA-2c. Therefore, the region between aa 1 and 70 of MSA-2c was not included in this alignment. Areas of amino acid identity are enclosed in black boxes, conservative substitutions have a gray background, and variant amino acid substitutions have a white background.
Msa-2a1, -2a2, -2b, and -2c genes encode ORFs of 317, 293, 288, and 266 amino acids (aa), respectively. A comparison of the predicted amino acid sequences of the msa-2a1, -2a2, and -2b genes is shown in Fig. 3B, and a comparison of MSA-2c and BabR 0.8 is shown in Fig. 3C. All four proteins share a nearly identical 22-aa carboxy terminus, a defining feature of all VMSA proteins that serves as a signal sequence for addition of a GPI moiety (10). As previously described by Hines et al. (10), there is a carboxy-terminal region of identity between MSA-2a1 and MSA-1 that extends upstream a further 13 aa. Interestingly, this 13-aa sequence (GNLQGQQGTTKPA [indicated by a boldface line over the sequence in Fig. 3B]) is conserved in MSA-2a2, but not in the other two members of the MSA-2 family. Additionally, MSA-2a1, -2a2, and -2b have an identical 39-aa amino terminus that includes a signal sequence for directing these proteins to the endoplasmic reticulum. The amino terminus of MSA-2c, while still consistent with a hydrophobic signal sequence (Vector NTI Suite 6), is dissimilar to all other known members of the VMSA family.
msa-2a1 and -2a2 have the highest level of nucleotide identity (88.9%), followed by the pairs msa-2a2/msa-2b (78.3%) and msa-2a1/msa-2b (71.5%). The msa-2c gene is the most divergent of the four msa-2-like genes in the Mo7 strain, and, as noted above, is most closely related to babr 0.8 (66% nucleotide identity). The nucleotide sequences of msa-2c and babr 0.8 show a relatively consistent level of identity throughout their length. In contrast, alignment of the predicted amino acid sequences of MSA-2c and BabR 0.8 (Fig. 3C) demonstrates three regions, each with a different degree of conservation as follows: (i) a relatively conserved N-terminal region (69% amino acid identity, 78% nucleotide identity), (ii) a central region of higher variability (40% amino acid identity, 51% nucleotide identity), and (iii) a carboxy-terminal region that, except for a common 4-aa ending segment (LSAF) is quite divergent (10% amino acid identity, 60% nucleotide identity). These results suggest that amino acid differences are a result of nucleotide deletions and/or insertions that have generated frameshifts without interrupting the ORF, consistent with the previous observations of Hines et al. (10).
In addition to the common sequences among the different VMSA genes identified above, there is a strictly conserved stretch of 54 bases in the 3′ untranslated region (3′-UTR) immediately following the end of the msa-1 and all of the msa-2 ORFs (data not shown). The homologous segment in the 3′-UTR downstream of the babr 0.8 ORF is 88.7% identical to the Mo7 msa-1 and msa-2 3′-UTRs (10).
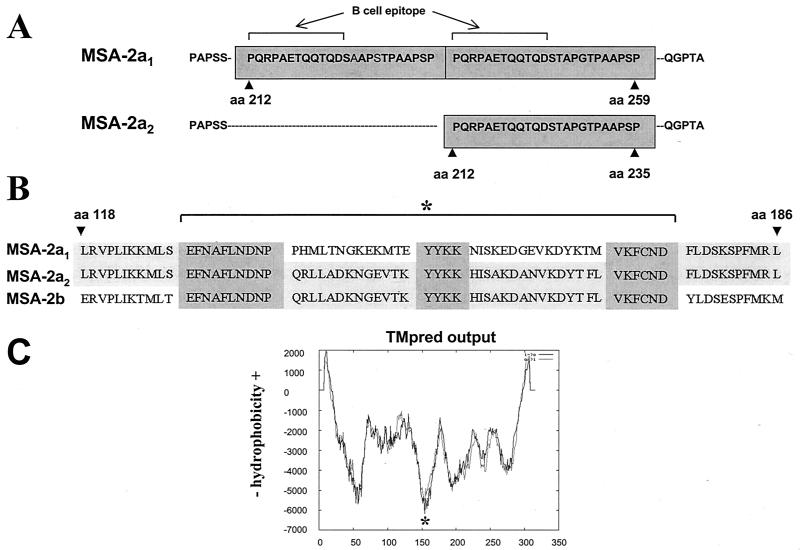
The MSA-2a1 and -2a2 amino acid sequences differ only in two regions (Fig. 4). First, MSA-2a1 contains a duplicated region between aa 212 and 259, previously shown to contain a surface-exposed B-cell epitope reacting with monoclonal antibody (MAb) 23/70.174 (12). In MSA-2a2, this repeat sequence is present only once between aa 212 and 235 (Fig. 4A). Second, the region containing aa 139 to 170 is divergent between MSA-2a1 and -2a2, but identical between MSA-2a2 and -2b, as depicted in Fig. 4B. This region is flanked by strictly conserved segments of 10 and 6 aa (amino and carboxy terminal, respectively) and contains a conserved YYKK central motif in all three (MSA-2a1, -2a2, and -2b) proteins. Interestingly, the area of apparent sequence exchange between MSA-2a2 and -2b coincides with a region of MSA-2 that is predicted to be strongly hydrophilic (Fig. 4C) and highly antigenic (Vector NTI Suite 6), suggesting that recombination has occurred in an area that is surface exposed and under selection pressure.
FIG. 4.
Detailed sequence comparison of MSA-2a1, -2a2, and -2b. (A) MSA-2a1 aa 207 to 264 and MSA-2a2 aa 207 to 240 are aligned to demonstrate changes in the repeat region. Amino acid repeats are enclosed in dark gray boxes. The location of the previously defined B-cell epitope (12) is shown. (B) MSA-2a1, -2a2, and -2b aa 118 to 186 are aligned to demonstrate the area of recombination (asterisk and bracket). Conserved regions among all three peptides that flank the recombination site and the conserved YYKK sequence are enclosed in dark gray boxes. (C) Secondary structure prediction of MSA-2a1. The predicted membrane orientation calculated with TMPred (http://www.ch.embnet.org/software/TMPRED_form.html) is plotted on the y axis against the amino acid position on the x axis. The solid line, i→o, is the calculation performed from N terminal to C terminal. The dashed line, o→i, is the calculation performed C terminal to N terminal. The most hydrophilic region of the molecule, which coincides with the area of recombination, is marked with an asterisk.
Transcription of Mo7 msa-2 genes.
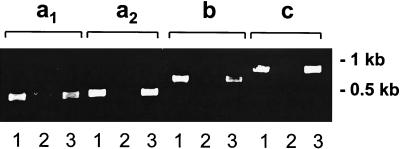
To determine whether all four msa-2 genes are transcribed, a specific set of primers was designed for each gene (Table 1) and used to amplify cDNA after reverse transcription of Mo7 mRNA. A single amplification product consistent with the predicted size was amplified from Mo7 cDNA and DNA, but not from mRNA when the RT step was eliminated (Fig. 5). Amplification products were cloned into pCR 2.1 and sequenced. In all cases, the sequences of the cDNA products were identical to the gene sequences obtained from pBS-msa2locus DNA (data not shown). These results demonstrate that all four msa-2 genes are transcribed in B. bovis Mo7 in vitro-cultured merozoites and confirm the absence of introns in the ORFs.
FIG. 5.
All four msa-2 genes are transcribed in erythrocytic stages of B. bovis Mo7. Ethidium bromide-stained agarose gel of amplicons of each msa-2 gene or transcript generated by using gene-specific primers (as detailed in Materials and Methods and Table 1) in PCR. Lanes 1, amplification of Mo7 cDNA after reverse transcription of mRNA; lanes 2, same as lane 1, without addition of RT; lanes 3, amplification of Mo7 DNA. Size markers are shown to the right.
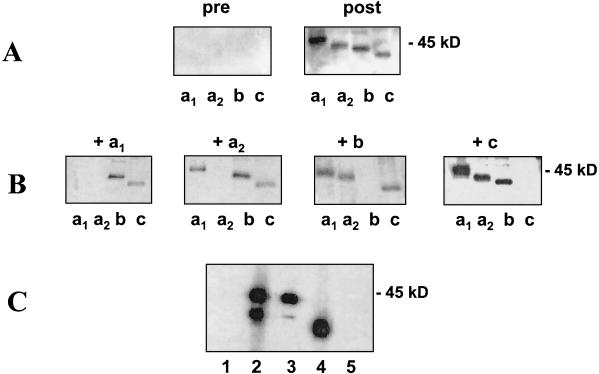
MSA-2 expression and immunogenicity.
In initial experiments to determine whether all four msa-2 genes are expressed and immunogenic, purified recombinant MSA-2a1 (rMSA-2a1), -2a2, -2b, and -2c were probed in immunoblots with bovine serum from an animal infected with the T2Bo B. bovis strain from Mexico (6, 19) (Fig. 6A). All rMSA-2 proteins were reactive with antibodies in postinfection serum. The presence of unique epitopes in each gene product was determined in competitive immunoblots by adding aliquots of each recombinant protein to immune serum during the incubations. The results are shown in Fig. 6B. Incubation of serum with rMSA-2b completely blocked the reaction of serum antibodies with itself, but did not eliminate reactivity with any of the other three MSA-2 antigens. Similarly, addition of rMSA-2c to serum during immunoblotting resulted in the specific abrogation of reactivity only against MSA-2c. In contrast, incubation of serum with rMSA-2a1 blocked antibody reactivity against both rMSA-2a1 and -2a2, but not rMSA-2b or -2c, while addition of rMSA-2a2 to serum during immunoblotting eliminated antibody reactivity against MSA-2a2, but not against rMSA-2a1, -2b, or -2c. These results confirm that MSA-2a1, -2b, and -2c are expressed and indicate that (i) MSA-2c contains unique B-cell epitopes, as predicted by the divergence of sequence between msa-2c and other genes in the locus; and (ii) MSA-2b contains B-cell epitopes not present in MSA-2a1 and -2a2, despite the high degree of sequence conservation among these proteins. The results also suggest that the insertion or deletion and recombination events that define the sequence differences between MSA-2a1 and -2a2 have not created unique B-cell epitopes in MSA-2a2. However, the results also are consistent with a lack of translation of the msa-2a2 transcript.
FIG. 6.
Reactivity of postinfection and monospecific serum antibodies against msa-2 gene products. (A) Immunoblots showing the reactivity of bovine preimmune and anti-Mo7 postinfection immune serum (1:500) against recombinant MSA-2 antigens. (B) Anti-Mo7 immune serum was blocked with each purified recombinant MSA-2 antigen as indicated, and the reactivity was tested against the different rMSA-2 antigens as in panel A. (C) Immunoblot showing the reactivity of MSA-2a1 monospecific, polyclonal serum antibodies and MAb 23/70.174 with native B. bovis merozoite antigens. Lane 1, control mouse serum; lane 2, polyclonal mouse serum against rMSA-2a1; lane 3, MAb 23/70.174; lane 4, polyclonal mouse serum against rMSA-2b run as a size marker; lane 5, control MAb Tryp1E1. The position of the 45-kDa molecular size marker is indicated on the right.
To further investigate MSA-2a2 expression, we determined whether MAb 23/70.174 against MSA-2a1 and murine monospecific polyclonal antibodies against rMSA-2a1 would react with native MSA-2a1 and MSA-2a2 in immunoblots (Fig. 6C). Monospecific serum antibodies (lane 2) bound to two proteins with molecular sizes consistent with those of MSA-2a1 and MSA-2a2. (MSA-2b is included as a relative size marker in lane 4.) Likewise, MAb 23/70.174 (which recognizes an MSA-2a1 epitope encoded by the 24-mer repeat sequence that is present but not duplicated in MSA-2a2 [Fig. 4A]) bound proteins with molecular sizes consistent with those of MSA-2a1 and MSA-2a2 (lane 3). Interestingly, the reactivity of MAb 23/70.174 is significantly greater against MSA-2a1 than that against MSA-2a2, suggesting that the presence of two contiguous 24-aa repeats in MSA-2a1 results in increased binding efficiency of the MAb. Polyclonal murine serum antibodies against rMSA-2b react with a single native merozoite protein consistent with the predicted molecular weight of MSA-2b (Fig. 6C, lane 4), further demonstrating that MSA-2b is expressed. In addition, the lack of cross-reactivity between rMSA-2a1 or rMSA-2a2 and rMSA-2b confirms the presence of unique B-cell epitopes in each protein and indicates that the 31-aa region common to MSA-2a2 and MSA-2b, while predicted to be highly antigenic, does not by itself comprise a B-cell epitope.
Conservation of msa-2 in an antigenically distinct B. bovis strain.
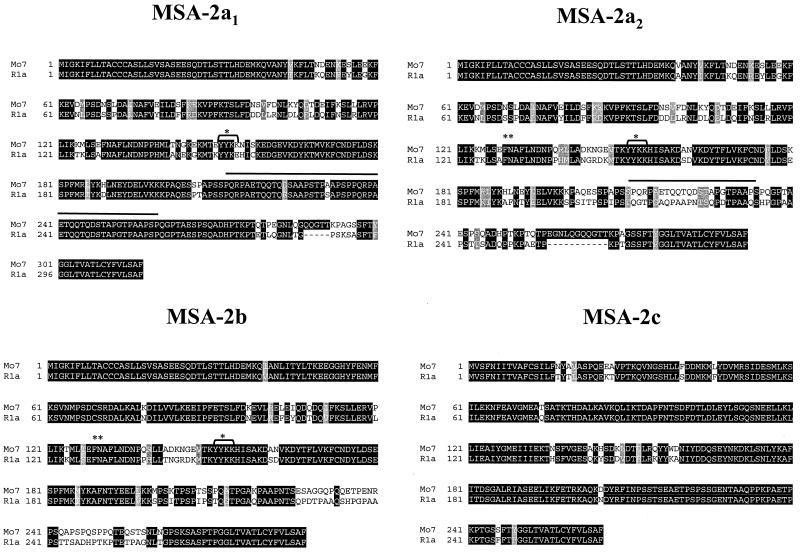
We investigated whether all four msa-2 genes were present in the antigenically divergent R1A B. bovis strain from Argentina (1) by amplification of msa-2a1, -2a2, and -2b with primers msa-2-F1 and B42/44R, and msa-2c with primers msa-2c-F and B42/44R (Table 1). All four msa-2 genes could be amplified from R1A DNA, and each of them was cloned and sequenced. The percentages of amino acid identity between respective MSA-2 homologues in these two strains are 83.6% for MSA-2a1, 69.4% for MSA-2a2, 79.1% for MSA-2b, and 88.7% for MSA-2c, a relatively high degree of conservation compared to the marked sequence divergence (52% identity) of MSA-1 proteins between Mo7 and R1A (26). Figure 7 shows alignments for each MSA-2 protein. Consistent with the pattern in all members of the VMSA family, the amino- and carboxy-terminal signal sequences are strictly conserved. The repeat sequence comprising the linear B-cell epitope in Mo7 MSA-2a1 recognized by MAb 23/70.174 (12) also is present twice in R1A MSA-2a1 (Fig. 7, bold line over sequence), with 2 aa changes (223Asp/Glu and 231Ala/Ser) in the first repeat. However, unlike Mo7 MSA-2a2, in which the same sequence is present once, in R1A MSA-2a2, the repeat sequence is not represented at all.
FIG. 7.
Amino acid conservation between MSA-2 peptides from the B. bovis Mo7 and R1A strains. DNA from the Argentina R1A strain was amplified by PCR with primers msa-2-F1 and B42/44R for the msa-2a1, -2a2, and -2b genes and msa-2c-F and B42/44R for msa-2c. Amplification products were cloned, and several clones were sequenced to identify R1A orthologues of msa-2a1, -2a2, -2b, and -2c based on the degree of sequence identity. The figure shows alignments for each gene product. Areas of amino acid identity are enclosed in black boxes, conservative amino acid substitutions have a gray background, and variant amino acids have a white background. Deletions are indicated with dashed lines. The repeat region in MSA-2a1 and -2a2 (bold line above sequence), conserved YYKK sequence (bracket and ∗), and recombination sites between MSA-2a2 and -2b (∗∗) are indicated.
Apparent recombination between R1A MSA-2a2 and MSA-2b has occurred at the same 5′ location as in the Mo7 msa-2 locus (Fig. 7, double asterisk), is flanked at the amino-terminal end by the same amino acids (EFNAFL) as Mo7, and contains the strictly conserved YYKK sequence (Fig. 7, asterisk; also see Fig. 4). Otherwise the recombined segment diverges in sequence and, in contrast to Mo7, extends downstream nearly to the conserved carboxy-terminal signal sequence.
DISCUSSION
In this study, four tandemly arranged and expressed msa-2-related genes were identified within a single genomic locus of the cloned B. bovis Mo7 strain. Of these four genes, only msa-2a1 (protein product originally termed Bo44) had been previously identified (12). However, the existence of multiple vmsa genes, including at least one additional paralogue of msa-2, had been suggested by Southern blot analysis with a probe containing the 3′-GPI anchor signal sequence, which is a strictly conserved feature of all members of the family (10, 12). In the previous studies with Mexico strain B. bovis DNA, five EcoRI fragments with approximate sizes of 1.08, 1.15, 2.2, 2.3, and 4.0 kb hybridized with a probe containing the conserved 3′ sequence. As indicated in the msa-2 locus map in Fig. 1B, EcoRI digestion of genomic Mo7 DNA should result in fragments of 2.3 kb (containing msa-2c), 1.15 kb (containing msa-2a1), 1.2 kb (containing msa-2a2), and >2 kb (containing msa-2b), which would hybridize with the 3′ probe used by Jasmer et al. (12). In addition, as previously reported (26), EcoRI digestion releases a 2.4-kb fragment containing the single-copy msa-1 gene. Therefore, the present results complete characterization of the five vmsa genes in the B. bovis Mo7 genome.
Phylogenetic analysis of the vmsa genes with the unrelated rap-1 gene used as an outlier suggests that the single-copy msa-1 gene has an ancestral relationship to other members of the family. At least two additional gene duplications followed by genetic divergence would create the tandemly arranged gene locus containing four related msa-2 genes. Of these, msa-2c is the most distantly related of the four and clusters separately with the babr 0.8 gene originally described in the Babesia rearranging (BabR) locus by Cowman et al. (3). The ORF of msa-2c starts 70 aa upstream of babr 0.8 as a result of a frameshift mutation in otherwise identical 5′ nucleotide sequences (data not shown). Frameshift mutations without introduction of a stop codon have previously been reported in the vmsa family (10) and may be a mechanism for generation of antigenic polymorphism among vmsa genes. Consistent with this observation, msa-2c and babr 0.8 nucleotide sequences are much more closely related than the amino acid sequences (Fig. 3C) as a result of mutations that maintain a different ORF. Evolutionary selection for this type of mutation in the vmsa family underscores its biological significance.
Southern blot analysis of genomic Mo7 DNA with a probe that hybridizes with the first 18 bp of the babr 0.8 ORF yielded a single 8.3-kb band (data not shown). Since the genes encoding the three BabR transcripts in the Australia K strain would hybridize with this probe, all babr-related genes in Mo7 appear to be contained in the msa-2 locus. Only one size of msa-2c transcript is present in cultured Mo7 merozoites. We are investigating whether different msa-2c-related transcripts occur in field isolates, as has been reported for BabR transcripts in the Australia K strain (3). However, the unique 5′ upstream regulatory region of msa-2c in comparison to the other three msa-2 genes in the locus supports the possibility of distinct transcriptional regulation of msa-2c.
A 54-bp 3′-UTR is conserved in all members of the vmsa family (10). This 3′-UTR contains a U-rich region and at least one putative polyadenylation signal (AATAA). Interestingly, the pentamer AATAA also is conserved in Plasmodium sp. parasites, where it acts as a polyadenylation signal (7, 14). The presence of 3′-UTR elements such as polyadenylation signals and U-rich regions contributes to control of expression of an ookinete protein of Plasmodium gallinaceum (7). Thus, the conservation of the 3′-UTR in all known members of the vmsa family of B. bovis suggests that this region may play a critical role in the expression of vmsa genes.
The divergence of msa-2a1, -2a2, and -2b genes is consistent with a combination of insertion or deletion of a repeat region and intergenic recombination. The apparent recombination between msa-2a2 and -2b has occurred at the same 5′ site in both Mo7 and R1A strains. While the sequence of the recombined segment is quite divergent between strains, the 5′ flanking sequence EFNAFL is conserved, suggesting that this sequence may define a hot spot for recombination. It is interesting that these changes have occurred either in known B-cell epitopes (the repeat region) or in a region predicted to be surface exposed and highly antigenic (the area of recombination). Immunologic analysis suggests that loss of one repeat in MSA-2a2 has resulted in a conformational change that alters the surface-exposed B-cell epitope bound by MAb 23/70.174. In contrast, the recombined segment of MSA-2a2 and -2b does not appear to result in immunologic cross-reactivity between these two proteins despite the prediction that this region is highly antigenic. Thus, if a B-cell epitope is present in this area, the recombined segment must contribute to it rather than define it.
All four msa-2-related genes are transcribed in vitro in the population of parasites comprising the Mo7 biological clone. Competitive immunoblots indicate that sequence differences among MSA-2a1, -2b, and -2c result in unique B-cell epitopes and that infected cattle respond to these epitopes. This finding also provides evidence that all three genes are expressed during the asexual, erythrocytic phase of the life cycle. The cross-reactivity of MSA-2a1 antibodies with a protein of the predicted size of MSA-2a2 in immunoblots also suggests that MSA-2a2 transcripts are expressed in merozoites. What is unclear, and of importance, is whether all four or some combination of VMSA proteins is coexpressed in an individual merozoite. MAbs against MSA-1 and -2a1 react with the entire population of merozoites in immunofluorescence assays (5, 20), indicating that, at a minimum, these two proteins are coexpressed in a single infected erythrocyte.
The exchange of domains among genes appears to be a common event among protozoal surface proteins and most likely occurs during sexual recombination in the vector. This results in mosaic polypeptides and is thought to be maintained and expanded through immunologic pressure and selection (8, 21, 22). A conserved flanking sequence can facilitate the exchange (21, 22). The presence of a strictly conserved 5′ sequence in msa-2a1, -2a2, and -2b of both Mo7 and R1A strains that flanks the site of recombination is consistent with this mechanism and appears to define one preferred site for recombination in msa-2 genes. The biological significance of the recombination event in B. bovis is unknown. However, it has occurred in an area corresponding to the most hydrophilic region of MSA-2a1 and contains a strictly conserved YYKK motif. Interestingly, a very similar motif, YFK, occurs in the same hydrophilic region of msa-1 and is the only sequence of 3 aa or more that is strictly conserved in all msa-1 genes from multiple Australia, Argentina, and Mexico vaccine and outbreak strains of B. bovis (26; McElwain et al., unpublished data). We postulate that this motif is essential for proper function of proteins encoded by the vmsa family.
Tandem arrangement of surface protein genes with short intergenic regions is common among protozoa and in babesial organisms was first described in Babesia rhodaini (25). Several plasmodial merozoite surface protein genes (msp-2, -4, and -5) that encode proteins that are vaccine candidates and that are postulated to be involved in merozoite invasion of erythrocytes are similarly arranged (13, 16, 17, 23, 24). The evolution of related, tandemly arranged surface protein genes in multiple hemoprotozoa indicates a selective advantage and functional significance. The role of these proteins in the merozoite membrane, particularly in relation to attachment and invasion of merozoites, is unknown. However, the ability of antibodies against them to block erythrocyte invasion provides solid evidence for their participation in host cell invasion. It is important for vaccine development to understand the molecular mechanisms underlying the coordinate function of tandemly arranged and expressed merozoite surface proteins in erythrocyte invasion. Just as important is an understanding of their expression, function, and genetic divergence in the vector. Studies with vmsa gene knockout and replacement constructs are under way to address these critical issues.
Acknowledgments
The skillful technical assistance of Deb Alperin and Bev Hunter is acknowledged. We thank Doug Jasmer and Lance Perryman for provision of MAb 23/70.174.
The work was supported by USAID PCE-G-00-98-00043-00, USDA NRI 96-35204-3667, and ARS CRIS 5348-32000-014-00D.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anziani, O. S., A. A. Guglielmone, A. A. Abdala, D. H. Aguirre, and A. J. Mangold. 1993. Proteccion conferida por Babesia bovis vacunal en novillos Holando Argentino. Rev. Med. Vet. (Buenos Aires) 74:47-49. [Google Scholar]
- 2.Brown, W. C., and G. H. Palmer. 1999. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol. Today 15:275-281. [DOI] [PubMed] [Google Scholar]
- 3.Cowman, A. F., O. Bernard, N. Stewart, and D. J. Kemp. 1984. Genes of the protozoan parasite Babesia bovis that rearrange to produce RNA species with different sequences. Cell 37:653-660. [DOI] [PubMed] [Google Scholar]
- 4.Falsenstein, J. 1993. PHYLIP (Phylogeny Inference Package) version 3.5c. Department of Genetics, University of Washington, Seattle. (Distributed by the author.)
- 5.Goff, W. L., W. C. Davis, G. H. Palmer, T. F. McElwain, W. C. Johnson, J. F. Bailey, and T. C. McGuire. 1988. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect. Immun. 56:2363-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff, W. L., W. C. Johnson, and C. W. Cluff. 1998. Babesia bovis immunity. In vitro and in vivo evidence for IL-10 regulation of IFN-gamma and iNOS. Ann. N. Y. Acad. Sci. 849:161-180. [DOI] [PubMed] [Google Scholar]
- 7.Golightly, L. M., W. Mbacham, J. Daily, and D. F. Wirth. 2000. 3′ UTR elements enhance expression of Pgs28, an ookinete protein of Plasmodium gallinaceum. Mol. Biochem. Parasitol. 105:61-70. [DOI] [PubMed] [Google Scholar]
- 8.Gubbels, M. J., F. Katzer, G. Hide, F. Jongejan, and B. R. Shiels. 2000. Generation of a mosaic pattern of diversity in the major merozoite-piroplasm surface antigen of Theileria annulata. Mol. Biochem. Parasitol. 110:23-32. [DOI] [PubMed] [Google Scholar]
- 9.Hines, S. A., T. F. McElwain, G. M. Buening, and G. H. Palmer. 1989. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol. Biochem. Parasitol. 37:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Hines, S. A., G. H. Palmer, D. P. Jasmer, T. C. McGuire, and T. F. McElwain. 1992. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 55:85-94. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman, K., and W. Stoffel. 1993. Tmbase—a database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 347:166. [Google Scholar]
- 12.Jasmer, D. P., D. W. Reduker, S. A. Hines, L. E. Perryman, and T. C. McGuire. 1992. Surface epitope localization and gene structure of a Babesia bovis 44-kilodalton variable merozoite surface antigen. Mol. Biochem. Parasitol. 55:75-83. [DOI] [PubMed] [Google Scholar]
- 13.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Immunization with recombinant Plasmodium yoelii merozoite surface protein 4/5 protects mice against lethal challenge. Infect. Immun. 68:6034-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzer, M., S. P. Wertheimer, D. de Bruin, and J. V. Ravetch. 1993. Plasmodium: control of gene expression in malaria parasites. Exp. Parasitol. 77:121-128. [DOI] [PubMed] [Google Scholar]
- 15.Levy, M. G., and M. Ristic. 1980. Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science 207:1218-1220. [DOI] [PubMed] [Google Scholar]
- 16.Marshall, V. M., A. Silva, M. Foley, S. Cranmer, L. Wang, D. J. McColl, D. J. Kemp, and R. L. Coppel. 1997. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect. Immun. 65:4460-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall, V. M., W. Tieqiao, and R. L. Coppel. 1998. Close linkage of three merozoite surface protein genes on chromosome 2 of Plasmodium falciparum. Mol. Biochem. Parasitol. 94:13-25. [DOI] [PubMed] [Google Scholar]
- 18.Palmer, G. H., and T. F. McElwain. 1995. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet. Parasitol. 57:233-253. [DOI] [PubMed] [Google Scholar]
- 19.Palmer, G. H., T. F. McElwain, L. E. Perryman, W. C. Davis, D. R. Reduker, D. P. Jasmer, V. Shkap, E. Pipano, W. L. Goff, and T. C. McGuire. 1991. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect. Immun. 59:3340-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reduker, D. W., D. P. Jasmer, W. L. Goff, L. E. Perryman, W. C. Davis, and T. C. McGuire. 1989. A recombinant surface protein of Babesia bovis elicits bovine antibodies that react with live merozoites. Mol. Biochem. Parasitol. 35:239-247. [DOI] [PubMed] [Google Scholar]
- 21.Rich, S. M., and F. J. Ayala. 2000. Population structure and recent evolution of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 97:6994-7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich, S. M., M. U. Ferreira, and F. J. Ayala. 2000. The origin of antigenic diversity in Plasmodium falciparum. Parasitol. Today 16:390-396. [DOI] [PubMed] [Google Scholar]
- 23.Saul, A., R. Lord, G. L. Jones, and L. Spencer. 1992. Protective immunization with invariant peptides of the Plasmodium falciparum antigen MSA2. J. Immunol. 148:208-211. [PubMed] [Google Scholar]
- 24.Smythe, J. A., R. L. Coppel, G. V. Brown, R. Ramasamy, D. J. Kemp, and R. F. Anders. 1988. Identification of two integral membrane proteins of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85:5195-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snary, D., and M. A. Smith. 1988. Sequence homology of surface membrane proteins of Babesia rodhaini. Mol. Biochem. Parasitol. 27:303-312. [DOI] [PubMed] [Google Scholar]
- 26.Suarez, C. E., M. Florin-Christensen, S. A. Hines, G. H. Palmer, W. C. Brown, and T. F. McElwain. 2000. Characterization of allelic variation in the Babesia bovis merozoite surface antigen 1 (MSA-1) locus and identification of a cross-reactive inhibition-sensitive MSA-1 epitope. Infect. Immun. 68:6865-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suarez, C. E., T. F. McElwain, I. Echaide, S. Torioni de Echaide, and G. H. Palmer. 1994. Interstrain conservation of babesial RAP-1 surface-exposed B-cell epitopes despite rap-1 genomic polymorphism. Infect. Immun. 62:3576-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suarez, C. E., G. H. Palmer, I. Hotzel, and T. F. McElwain. 1998. Structure, sequence, and transcriptional analysis of the Babesia bovis rap-1 multigene locus. Mol. Biochem. Parasitol. 93:215-224. [DOI] [PubMed] [Google Scholar]
- 29.Visser, E. S., T. C. McGuire, G. H. Palmer, W. C. Davis, V. Shkap, E. Pipano, and D. P. Knowles, Jr. 1992. The Anaplasma marginale msp5 gene encodes a 19-kilodalton protein conserved in all recognized Anaplasma species. Infect. Immun. 60:5139-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]