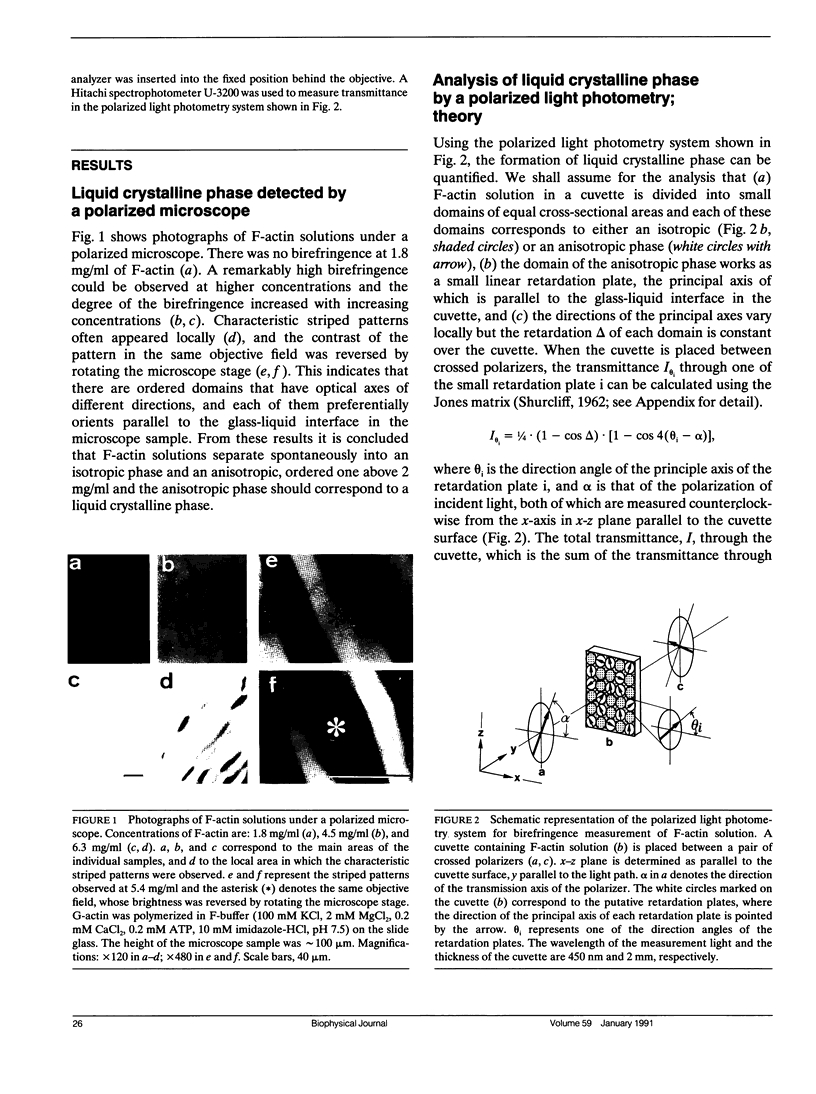
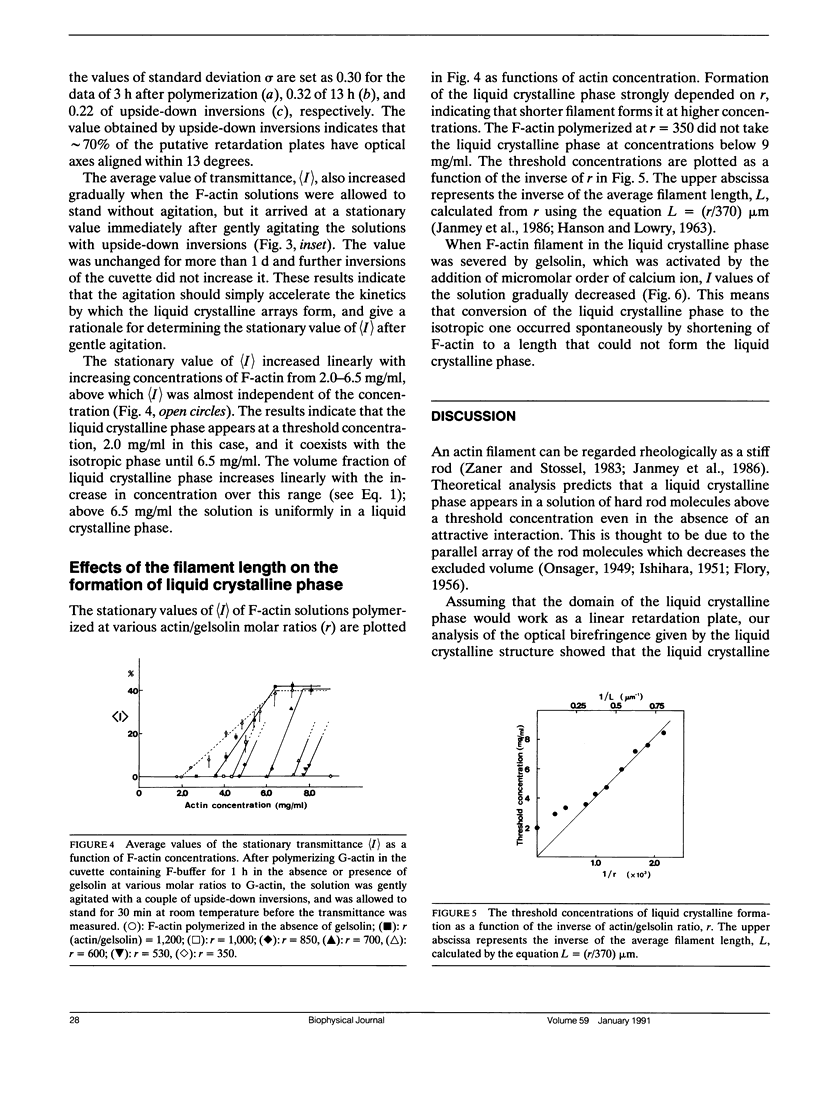
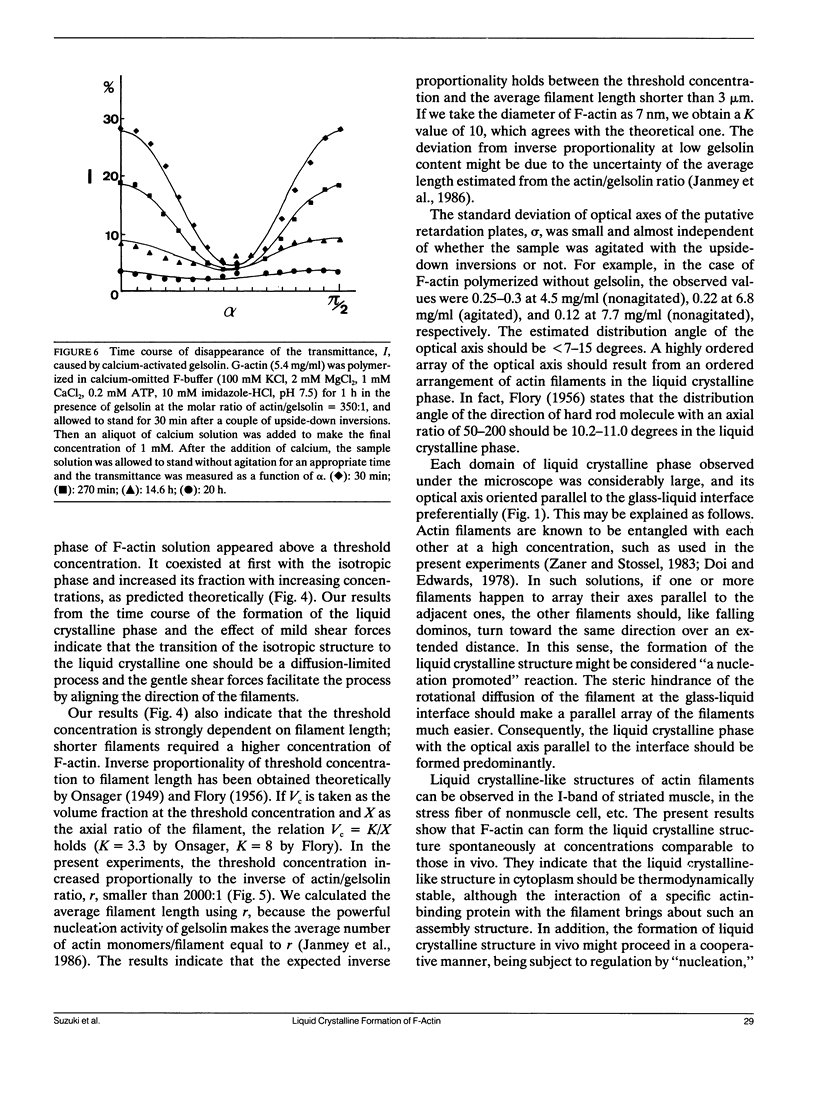
Abstract
We studied the formation and structure of liquid crystalline phase of F-actin solutions by polarized light photometry, assuming that a small domain of the liquid crystalline phase works as a linear retardation plate. Transmittance of polarized light due to the birefringence of liquid crystalline phase appeared above a threshold concentration of F-actin. The threshold increased with a decrease in filament length, which was regulated by calcium-activated gelsolin. The intensity increased linearly with increasing concentrations until it reached a stationary value. The deviation of optical axis direction of the putative retardation plate was estimated 7-15 degrees. These results indicate that:(a) the liquid crystalline phase is formed above a threshold concentration of F-actin; (b) the threshold is proportional to the inverse of filament length; (c) the ordered phase coexists with the isotropic one, increasing the volume fraction with increasing concentrations until all filaments take the liquid crystalline structure; (d) the filaments in liquid crystalline phase take a highly ordered array. These results can be attributed to the excluded volume effect of rod-like molecules on the formation of liquid crystalline structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doi Y., Higashida M., Kido S. Plasma-gelsolin-binding sites on the actin sequence. Eur J Biochem. 1987 Apr 1;164(1):89–94. doi: 10.1111/j.1432-1033.1987.tb10997.x. [DOI] [PubMed] [Google Scholar]
- Hanson J. Evidence from electron microscope studies on actin paracrystals concerning the origin of the cross-striation in the thin filaments of vertebrate skeletal muscle. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):39–58. doi: 10.1098/rspb.1973.0003. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Peetermans J., Zaner K. S., Stossel T. P., Tanaka T. Structure and mobility of actin filaments as measured by quasielastic light scattering, viscometry, and electron microscopy. J Biol Chem. 1986 Jun 25;261(18):8357–8362. [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Polymorphism of F-actin. I. Three forms of paracrystals. J Biochem. 1970 Dec;68(6):885–899. doi: 10.1093/oxfordjournals.jbchem.a129428. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Contribution of actin to the structure of the cytoplasmic matrix. J Cell Biol. 1984 Jul;99(1 Pt 2):15s–21s. doi: 10.1083/jcb.99.1.15s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelecka T. E., Davidson M. W., Rill R. L. Multiple liquid crystal phases of DNA at high concentrations. Nature. 1988 Feb 4;331(6155):457–460. doi: 10.1038/331457a0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Yamazaki M., Ito T. Osmoelastic coupling in biological structures: formation of parallel bundles of actin filaments in a crystalline-like structure caused by osmotic stress. Biochemistry. 1989 Jul 25;28(15):6513–6518. doi: 10.1021/bi00441a052. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Iida K., Janmey P. A. Identification of a polyphosphoinositide-modulated domain in gelsolin which binds to the sides of actin filaments. J Cell Biol. 1988 Mar;106(3):805–812. doi: 10.1083/jcb.106.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaner K. S., Stossel T. P. Physical basis of the rheologic properties of F-actin. J Biol Chem. 1983 Sep 25;258(18):11004–11009. [PubMed] [Google Scholar]



