Abstract
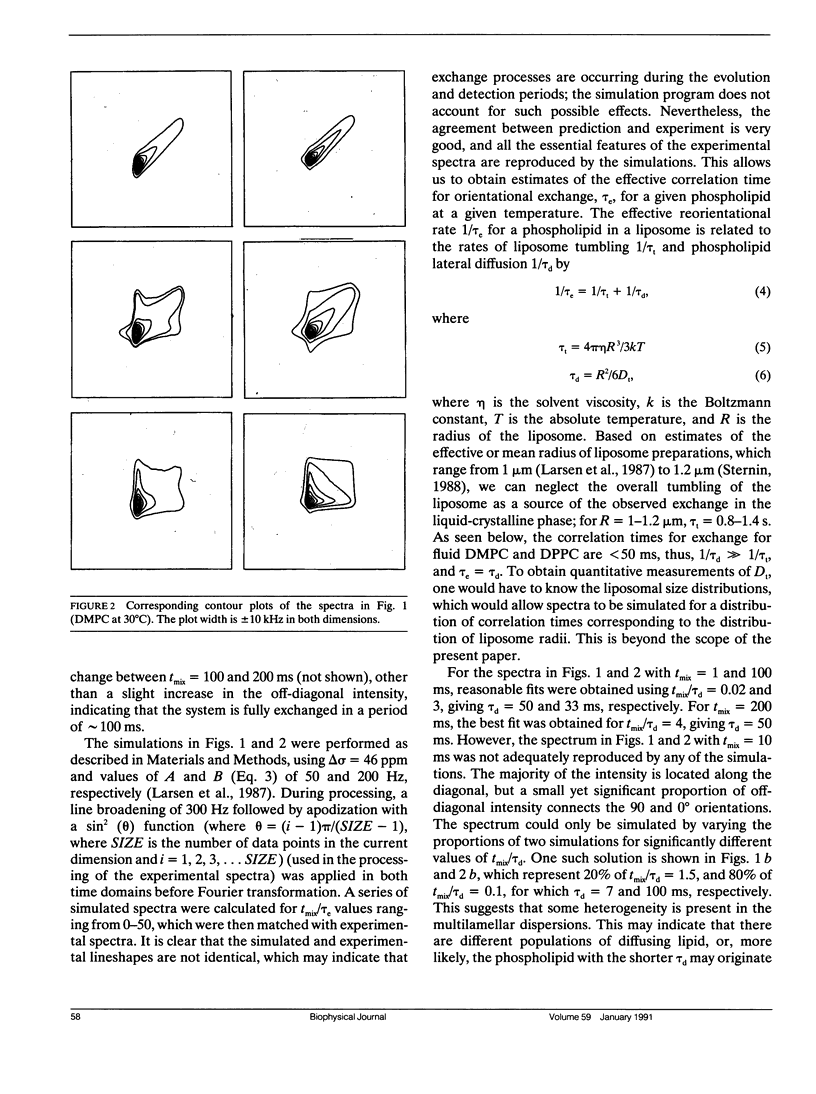
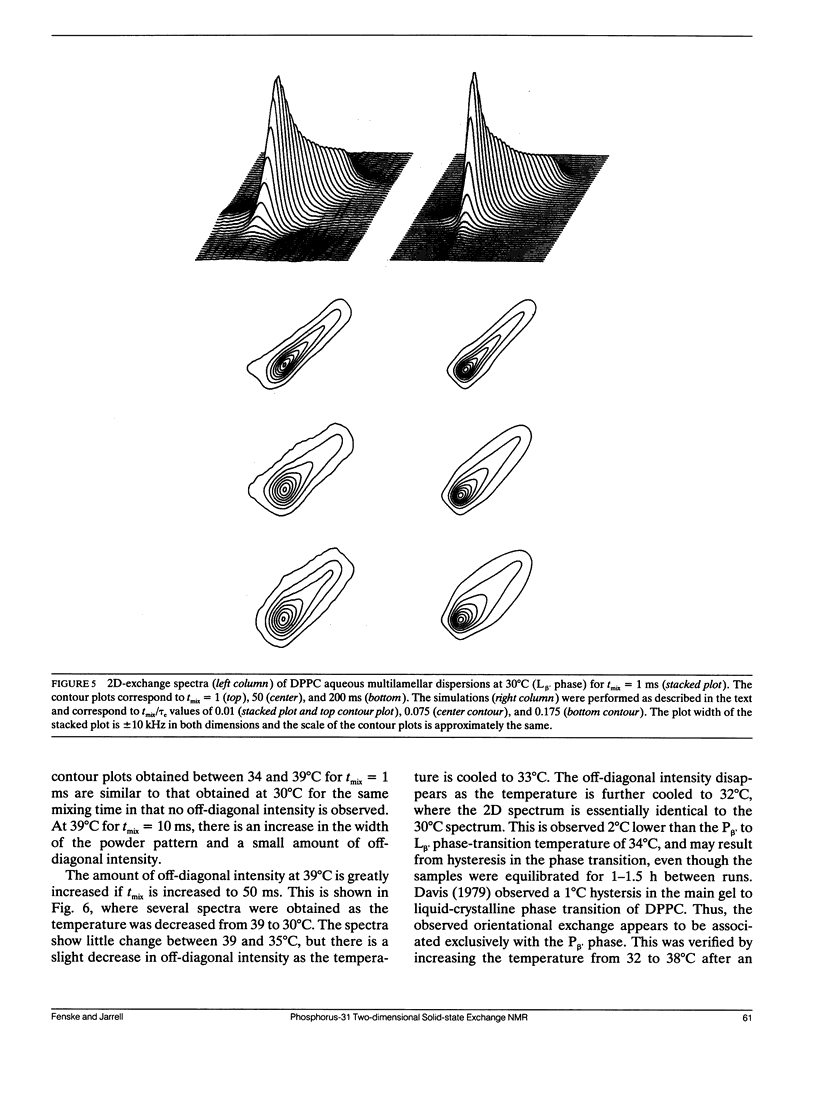
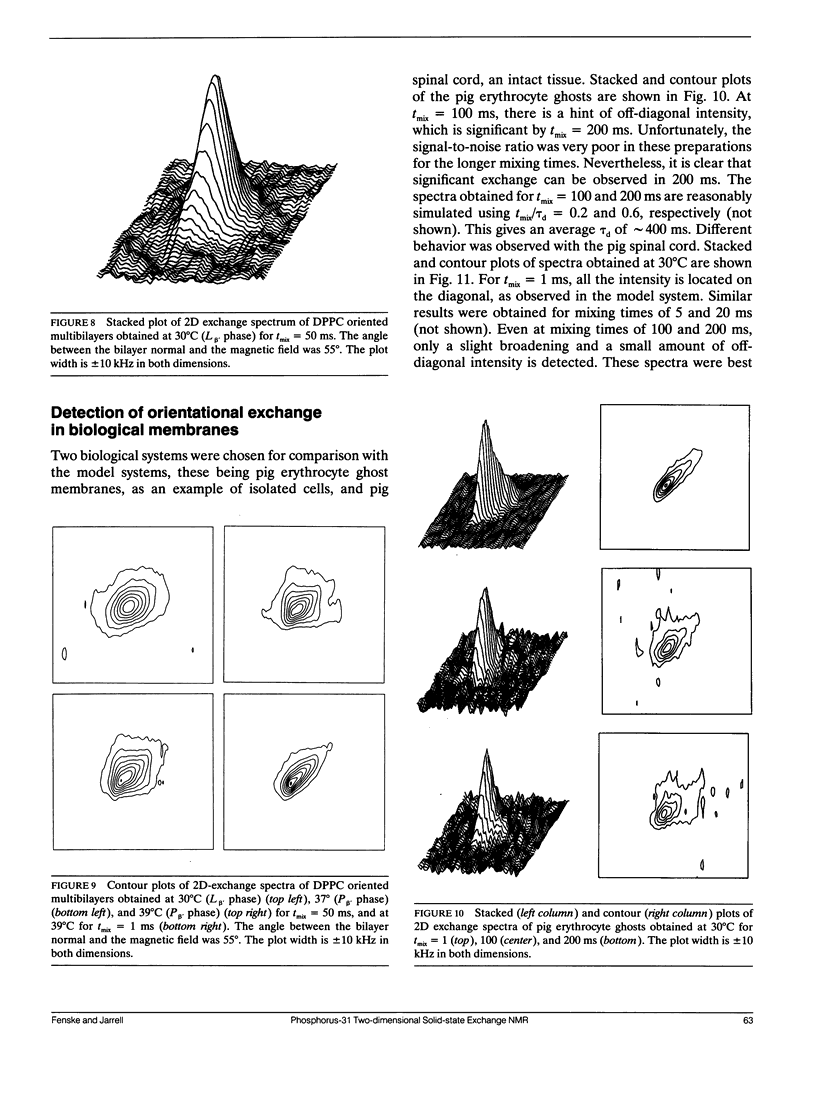
Two-dimensional solid-state 31P NMR has been used to investigate the orientational exchange of phospholipids in gel and liquid-crystalline aqueous multilamellar dispersions and oriented multibilayers, and in biological membranes. In liquid-crystalline L alpha multilamellar dispersions, orientational exchange originates from the lateral diffusion of phospholipid molecules over the curved surface of the liposomes and is manifest by an increase in off-diagonal intensity, which correlates the 90 and 0 degrees orientations of the membrane normal with respect to the magnetic field when the system is fully exchanged. Spectral simulations of the time evolution of exchange allowed determination of the correlation times tau d for lateral diffusion. For DMPC and DPPC at comparable reduced temperatures, tau d values of 44 and 8 ms were obtained, respectively. The nature and rate of exchange observed for POPE at 30 degrees C is similar to that of DMPC at the same temperature. The measured correlation times are consistent with diffusion rates obtained by FRAP for liposomes with radii in the 1 micron range. In the gel phase of DPPC (30 degrees C), little orientational exchange is observed at mixing times up to 200 ms, demonstrating that the lateral diffusion is very slow. The correlation time for orientational exchange obtained from spectral simulations was approximately 900 ms; thus, exchange in the gel state is at least two orders of magnitude slower than in the liquid-crystalline state. In the P beta (ripple) phase, at temperatures between 34 and 39 degrees C, significant exchange is observed for mixing times between 50 and 200 ms. Exchange is also observed in oriented samples of DPPC in the P beta phase for mixing times of 50 ms, but not for oriented liquid-crystalline samples for mixing times up to 100 ms. The exchange observed in the ripple phase could originate from rapid lateral diffusion of "fast" diffusing phospholipid within defect structures, and/or from "slow" lateral diffusion of ordered phospholipid over the ripples. 2D experiments were also performed on pig erythrocyte ghosts and on intact pig spinal cord. Significant orientational exchange was observed with the erythrocyte ghosts at a mixing time of 200 ms, but almost no exchange was observed with the spinal cord at the same mixing time. Spectral simulations suggest tau d values of approximately 400 ms and 1.3 s for the erythrocyte ghosts and spinal cord at 30 degrees C. The results demonstrate that exchange in the biological membranes is significantly slower than in the model membrane systems, which suggests that the cell surfaces are relatively "smooth," i.e., any local surface perturbations are either present in small number or have little effect on the mean orientation of the phospholipids with respect to the membrane normal.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell M. F., Gounaris K., Zara S. J., Barber J. A method for estimating lateral diffusion coefficients in membranes from steady-state fluorescence quenching studies. Biophys J. 1987 May;51(5):735–744. doi: 10.1016/S0006-3495(87)83400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Davis J. H. Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys J. 1979 Sep;27(3):339–358. doi: 10.1016/S0006-3495(79)85222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Jarrell H. C., Jovall P. A., Giziewicz J. B., Turner L. A., Smith I. C. Determination of conformational properties of glycolipid head groups by 2H NMR of oriented multibilayers. Biochemistry. 1987 Apr 7;26(7):1805–1811. doi: 10.1021/bi00381a003. [DOI] [PubMed] [Google Scholar]
- Kapitza H. G., Rüppel D. A., Galla H. J., Sackmann E. Lateral diffusion of lipids and glycophorin in solid phosphatidylcholine bilayers. The role of structural defects. Biophys J. 1984 Mar;45(3):577–587. doi: 10.1016/S0006-3495(84)84195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay A. L., Burnell E. E., Nichol C. P., Weeks G., Bloom M., Valic M. I. Effect of viscosity on the width of the methylene proton magnetic resonance line in sonicated phospholipid bilayer vesicles. FEBS Lett. 1978 Apr 1;88(1):97–100. doi: 10.1016/0014-5793(78)80616-4. [DOI] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P angular dependent nuclear spin relaxation time study. Biophys J. 1989 Sep;56(3):543–549. doi: 10.1016/S0006-3495(89)82701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P nuclear relaxation time study. Biophys J. 1987 Nov;52(5):791–799. doi: 10.1016/S0006-3495(87)83273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. J., Luxnat M., Galla H. J. Lateral diffusion of small solutes and partition of amphipaths in defect structures of lipid bilayers. Biochim Biophys Acta. 1986 Apr 14;856(2):283–289. doi: 10.1016/0005-2736(86)90038-6. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. The effect of mobile obstacles. Biophys J. 1987 Dec;52(6):989–997. doi: 10.1016/S0006-3495(87)83291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. B., Chan W. K., Webb W. W. Fast diffusion along defects and corrugations in phospholipid P beta, liquid crystals. Biophys J. 1983 Aug;43(2):157–165. doi: 10.1016/S0006-3495(83)84336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J., Tamm L., Hymel L., Fleischer S. Deuterium and phosphorus nuclear magnetic resonance and fluorescence depolarization studies of functional reconstituted sarcoplasmic reticulum membrane vesicles. Biochemistry. 1981 Jun 23;20(13):3922–3932. doi: 10.1021/bi00516a040. [DOI] [PubMed] [Google Scholar]
- Stamatoff J., Feuer B., Guggenheim H. J., Tellez G., Yamane T. Amplitude of rippling in the P beta phase of dipalmitoylphosphatidylcholine bilayers. Biophys J. 1982 Jun;38(3):217–226. doi: 10.1016/S0006-3495(82)84551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne J. F., Dupou-Cézanne L., Lopez A., Tournier J. F. Lipid lateral diffusion and membrane organization. FEBS Lett. 1989 Oct 23;257(1):10–16. doi: 10.1016/0014-5793(89)81774-0. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- Wack DC, Webb WW. Synchrotron x-ray study of the modulated lamellar phase P beta ' in the lecithin-water system. Phys Rev A Gen Phys. 1989 Sep 1;40(5):2712–2730. doi: 10.1103/physreva.40.2712. [DOI] [PubMed] [Google Scholar]


