Abstract
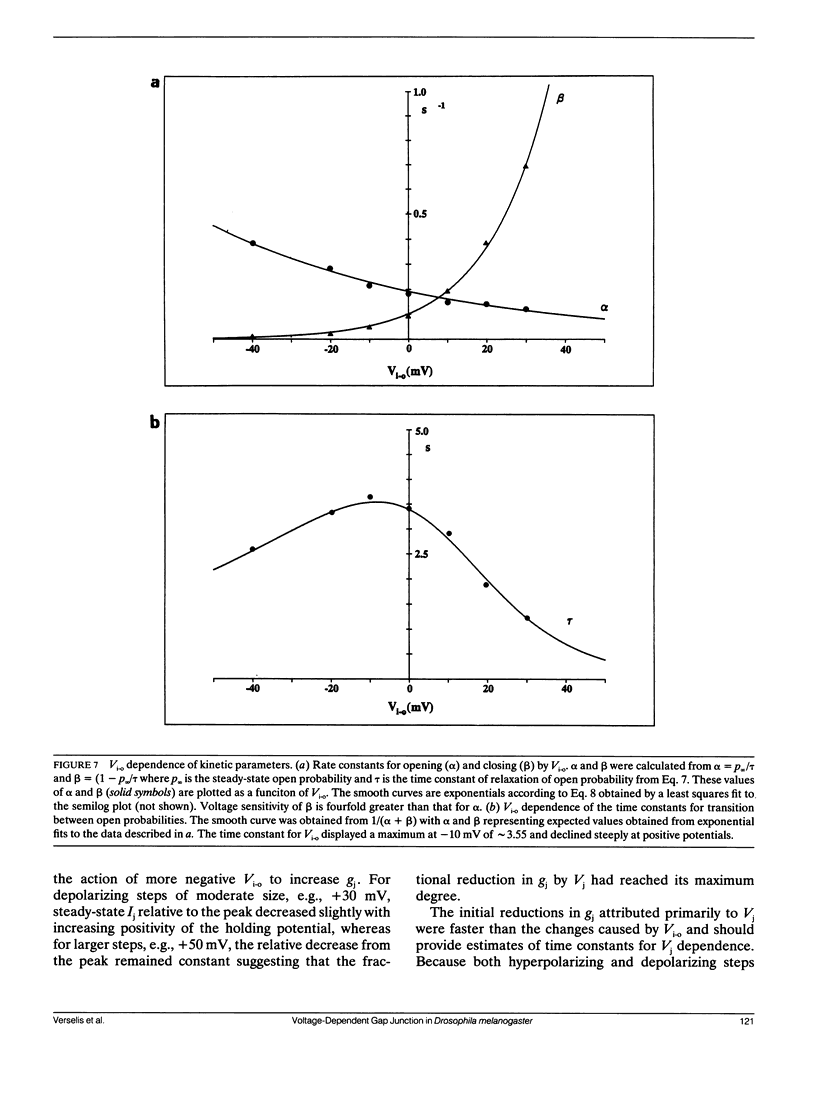
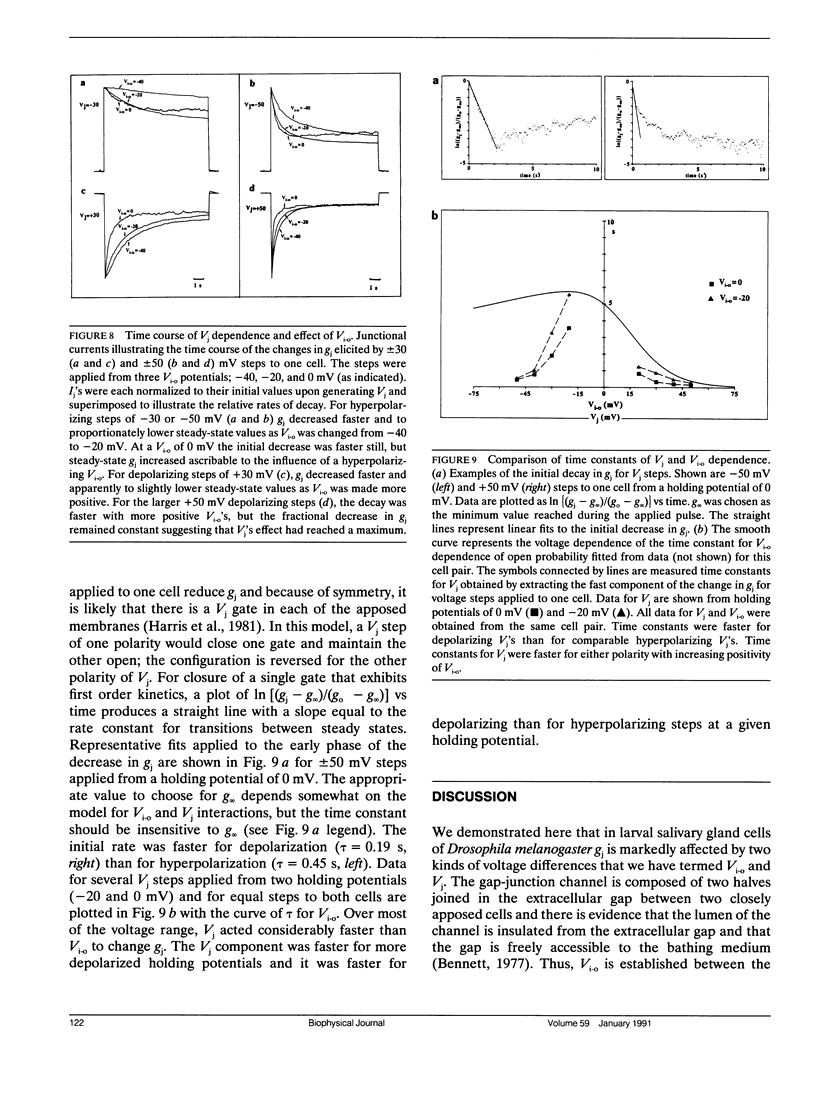
Steady-state and kinetic analyses of gap junctional conductance, gi, in salivary glands of Drosophila melanogaster third instar larvae reveal a strong and complex voltage dependence that can be elicited by two types of voltages. Voltages applied between the cells, i.e., transjunctional voltages, Vj, and those applied between the cytoplasm and the extracellular space, inside-outside voltages, Vi,o, markedly alter gj. Alteration of Vi-o while holding Vj = O,i.e., by equal displacement of the voltages in the cells, causes gj to increase to a maximum on hyperpolarization and to decrease to near zero on depolarization. These conductance changes associated with Vi-o are fit by a model in which there are two independent gates in series, one in each series, one in each membrane, where each gate is equally sensitive to Vi-o and exhibits first order kinetics. Vj's generated by applying voltage steps of either polarity to either cell, substantially reduce gj. These conductance changes exhibit complex kinetics that depend on Vi-o as well as Vj. At more positive Vi-o's, the changes in gj have two phases, an early phase consisting of of a decrease in gj for either polarity of Vj and a later phase consisting of an increase in gj on hyperpolarizing either cell and a decrease on depolarizing either cell. At negative Vi-o's in the plateau region of the gj-Vi-o relation, the later slow increase in gj is absent on hyperpolarizing either cell. Also, the early decrease in gj for either polarity of Vj is faster the more positive the Vi-o. The complex time course elicited by applying voltage steps to one cell can be explained as combined actions of Vi-o and Vj, with the early phase ascribable to Vj, but influenced by Vi-o, and the later phase to the changes in Vi-o associated with the generation of Vj. The substantially different kinetics and sensitivity of changes in gj by Vi-o and Vj suggests that the mechanisms of gating by these two voltages are different. Evidently, these gap-junction channels are capable of two distinct, but interactive forms of voltage dependence.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A. A., Bennett M. V. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969 Feb;53(2):211–237. doi: 10.1085/jgp.53.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Beyer E. C., Swenson K. I., Paul D. L., Goodenough D. A. Cloning and expression of a Xenopus embryonic gap junction protein. Science. 1989 Mar 3;243(4895):1194–1195. doi: 10.1126/science.2466337. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimlich R. L., Kumar N. M., Gilula N. B. Sequence and developmental expression of mRNA coding for a gap junction protein in Xenopus. J Cell Biol. 1988 Sep;107(3):1065–1073. doi: 10.1083/jcb.107.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Control of intercellular communication by voltage dependence of gap junctional conductance. J Neurosci. 1983 Jan;3(1):79–100. doi: 10.1523/JNEUROSCI.03-01-00079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaslove S. W., Brink P. R. The mechanism of rectification at the electrotonic motor giant synapse of the crayfish. Nature. 1986 Sep 4;323(6083):63–65. doi: 10.1038/323063a0. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Ramón F. Voltage independence of an electrotonic synapse. Biophys J. 1982 Jul;39(1):115–117. doi: 10.1016/S0006-3495(82)84497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986 Sep;103(3):767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta J. F., Walcott B. Conductance and dye permeability of a rectifying electrical synapse. Nature. 1983 Sep 1;305(5929):52–55. doi: 10.1038/305052a0. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Obaid A. L., Socolar S. J., Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane potential, calcium, and pH. J Membr Biol. 1983;73(1):69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Gallacher D. V. Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- Rook M. B., Jongsma H. J., van Ginneken A. C. Properties of single gap junctional channels between isolated neonatal rat heart cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H770–H782. doi: 10.1152/ajpheart.1988.255.4.H770. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Verselis V., Brink P. R. Voltage clamp of the earthworm septum. Biophys J. 1984 Jan;45(1):147–150. doi: 10.1016/S0006-3495(84)84143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis V., White R. L., Spray D. C., Bennett M. V. Gap junctional conductance and permeability are linearly related. Science. 1986 Oct 24;234(4775):461–464. doi: 10.1126/science.3489990. [DOI] [PubMed] [Google Scholar]
- White R. L., Spray D. C., Campos de Carvalho A. C., Wittenberg B. A., Bennett M. V. Some electrical and pharmacological properties of gap junctions between adult ventricular myocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C447–C455. doi: 10.1152/ajpcell.1985.249.5.C447. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]