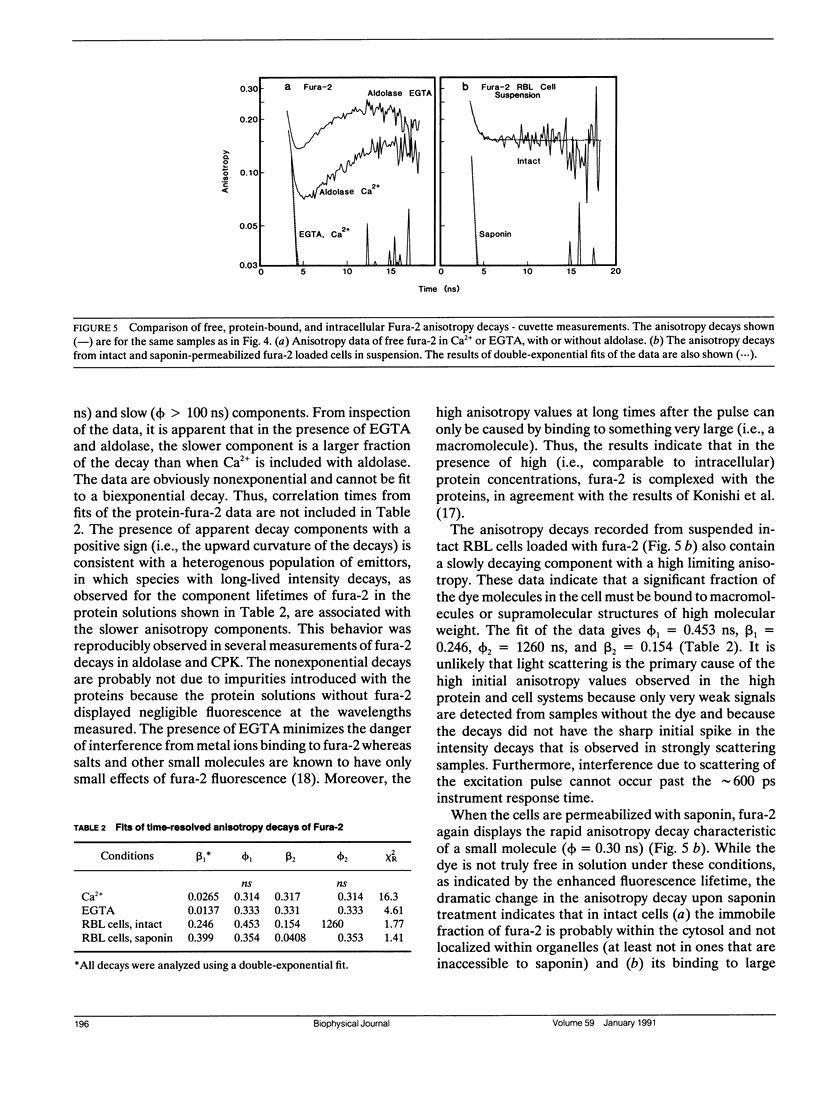
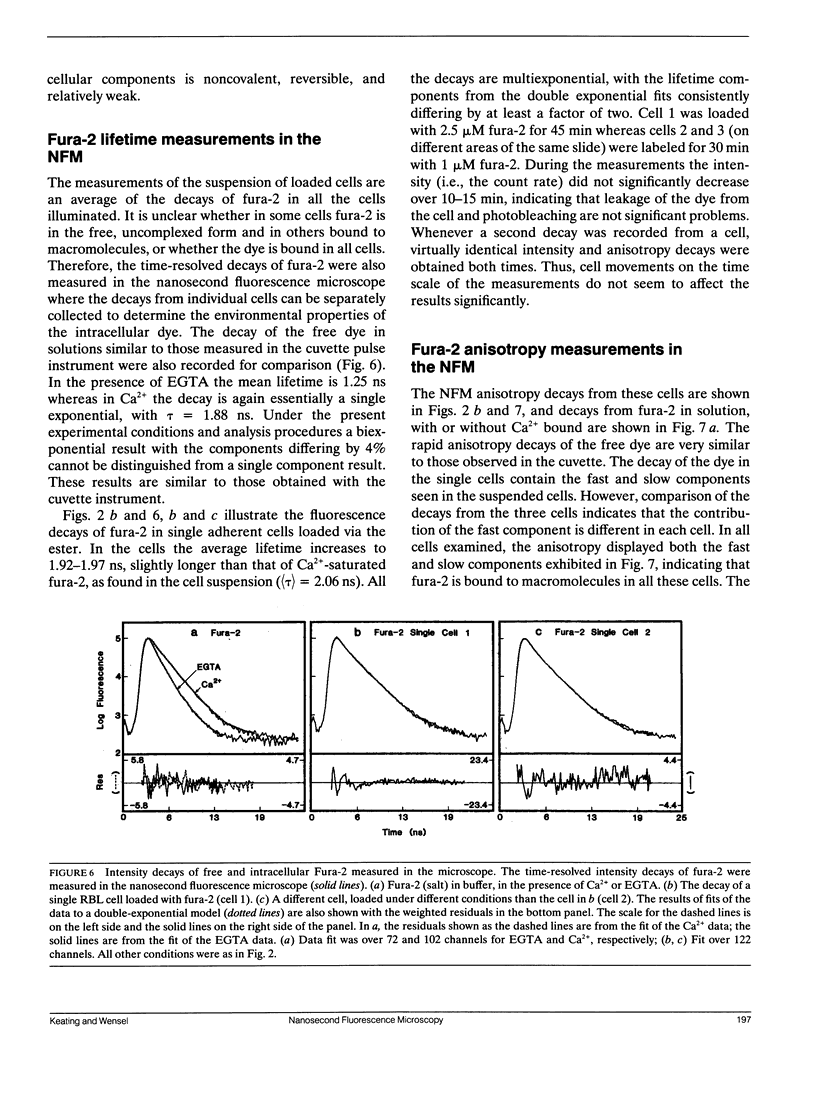
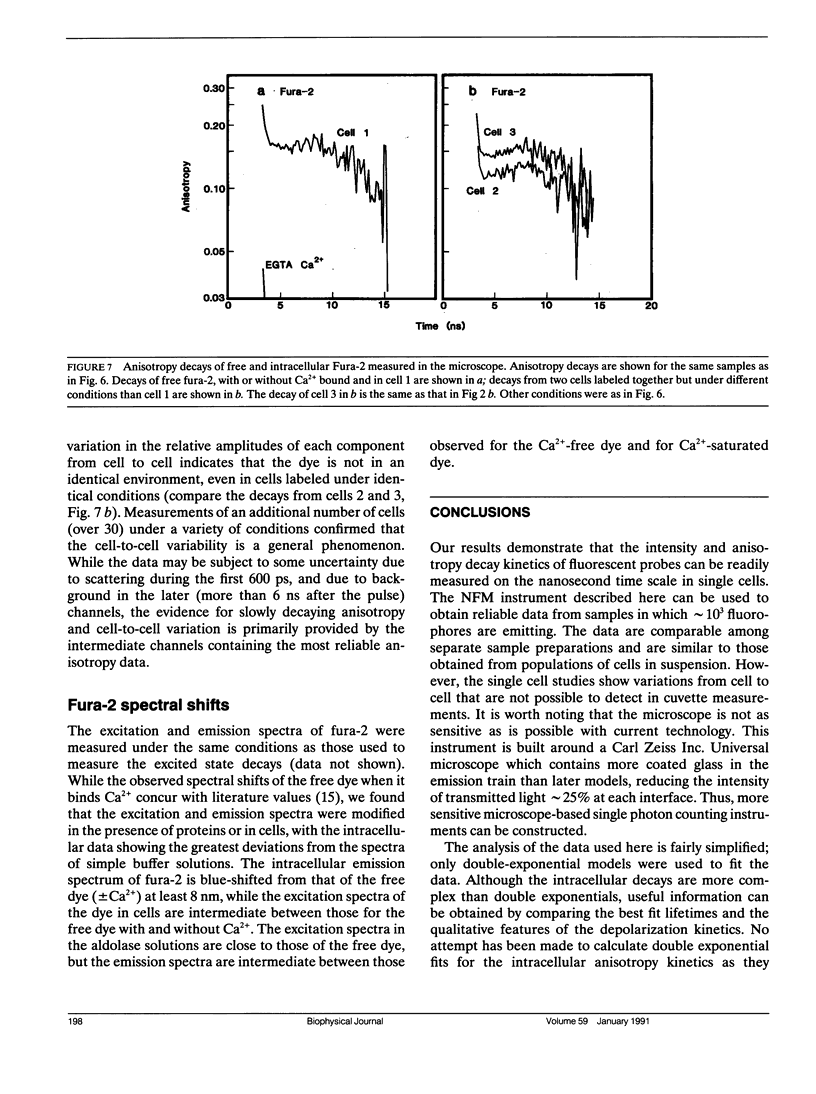
Abstract
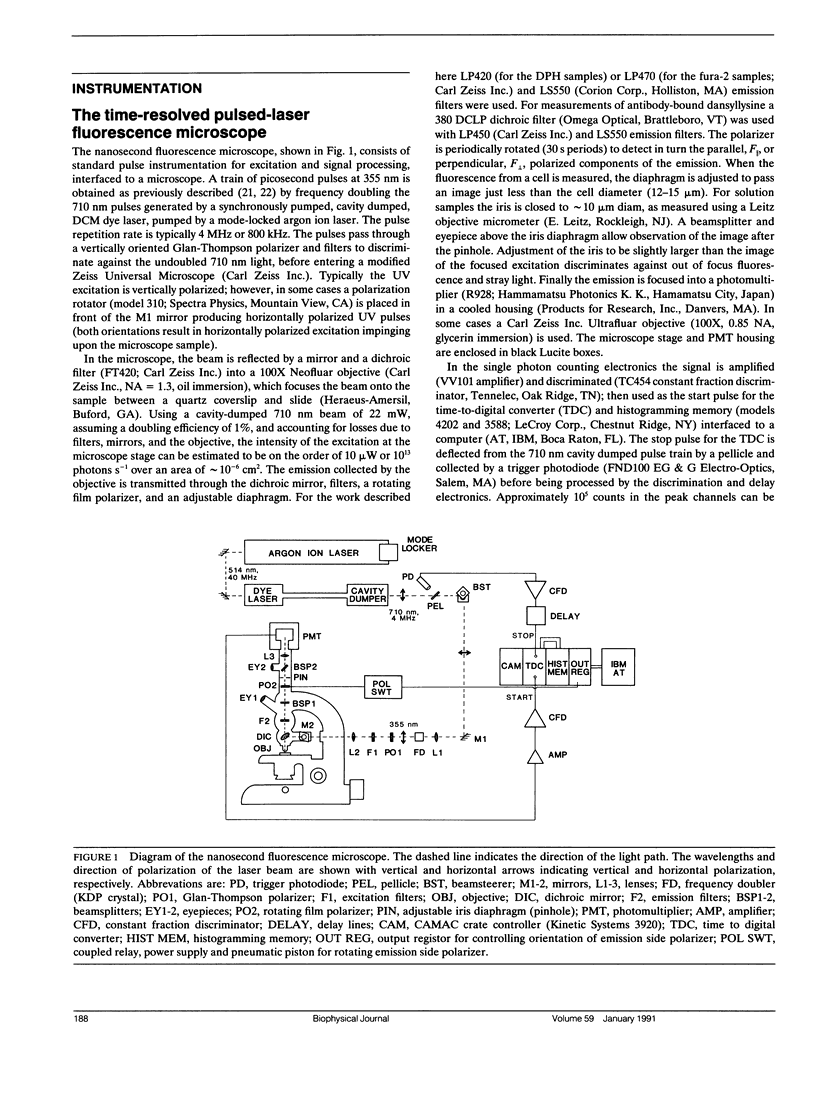
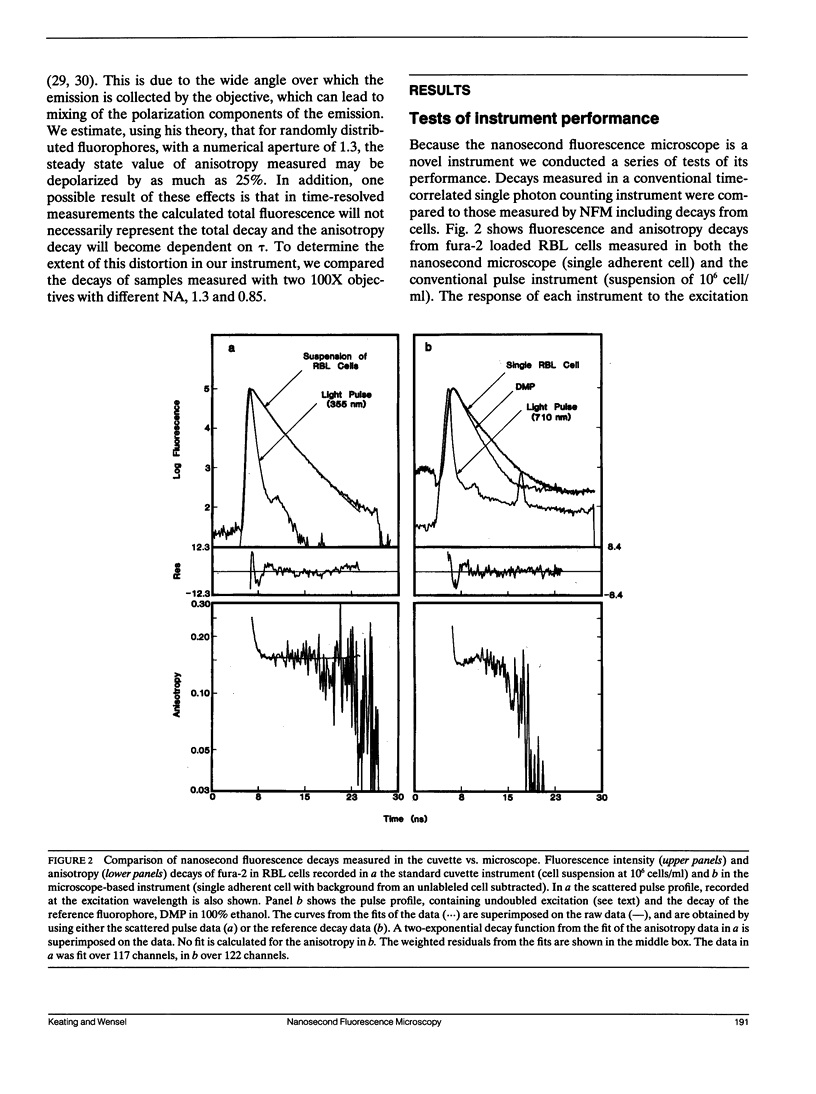
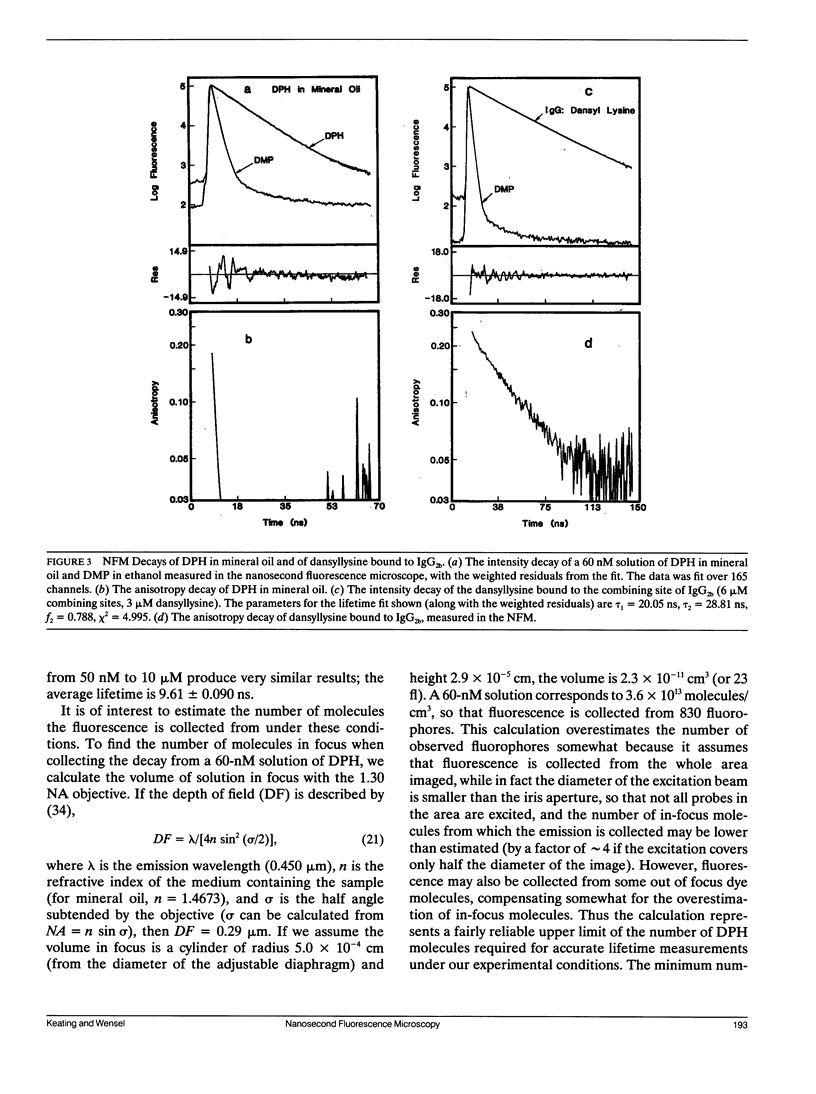
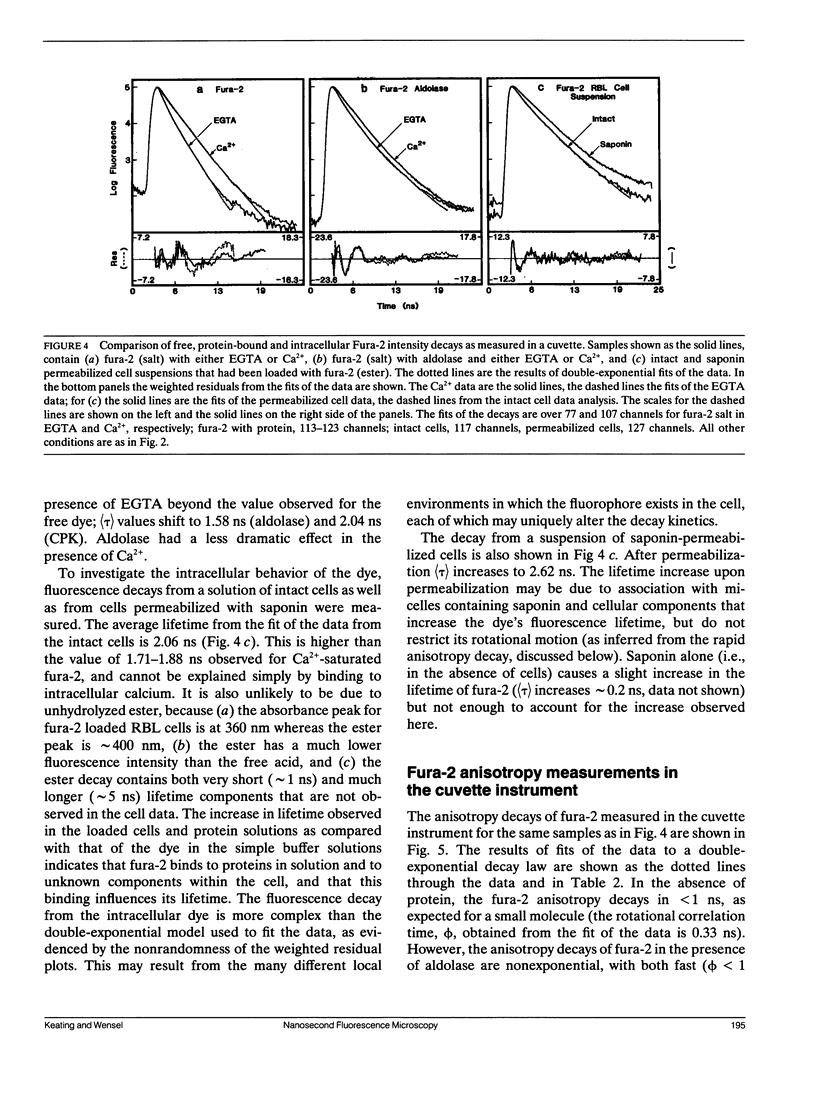
A microscope based time-correlated single photon counting instrument has been constructed to measure fluorescence intensity and emission anisotropy decays from fluorophores in single cells on a nanosecond time scale. The sample is excited and the emission collected using epi-illumination optics with frequency-doubled pulses from the cavity-dumped output of a synchronously pumped dye laser serving as an excitation source. Collection of decays from a single cell is possible due to the presence of an iris in the emission path that can be reduced to less than the diameter of a single cell. Using the instrument the decay of 60 nM 1,6-diphenyl-1,3,5-hexatriene was measured, demonstrating that adequate data for lifetime analysis can be recorded from fewer 10(3) molecules of the fluorophore in an illuminated volume of 23 fl. In addition, the intensity and anisotropy decays of fura-2 in single adherent cells and in suspensions of fura-2 loaded cells in suspension, although the relative amplitudes and decay constants vary somewhat from cell to cell. The results indicate that a significant but variable fraction of fura-2 is bound to relatively immobile macromolecular components in these cells.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Mohanna F. A., Hallett M. B. The use of fura-2 to determine the relationship between cytoplasmic free Ca2+ and oxidase activation in rat neutrophils. Cell Calcium. 1988 Feb;9(1):17–26. doi: 10.1016/0143-4160(88)90034-6. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization. Biophys J. 1979 Jun;26(3):557–573. doi: 10.1016/S0006-3495(79)85271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Fluorescence polarization microscopy. Methods Cell Biol. 1989;30:333–352. [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beechem J. M., Brand L. Time-resolved fluorescence of proteins. Annu Rev Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Chen L. A., Brand L. Rotational relaxation of the "microviscosity" probe diphenylhexatriene in paraffin oil and egg lecithin vesicles. J Biol Chem. 1977 Nov 10;252(21):7500–7510. [PubMed] [Google Scholar]
- Dangl J. L., Wensel T. G., Morrison S. L., Stryer L., Herzenberg L. A., Oi V. T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988 Jul;7(7):1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docchio F., Ramponi R., Sacchi C. A., Bottiroli G., Freitas I. An automatic pulsed laser microfluorometer with high spatial and temporal resolution. J Microsc. 1984 May;134(Pt 2):151–160. doi: 10.1111/j.1365-2818.1984.tb02504.x. [DOI] [PubMed] [Google Scholar]
- Gratton E., Limkeman M., Lakowicz J. R., Maliwal B. P., Cherek H., Laczko G. Resolution of mixtures of fluorophores using variable-frequency phase and modulation data. Biophys J. 1984 Oct;46(4):479–486. doi: 10.1016/S0006-3495(84)84044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Kolber Z. S., Barkley M. D. Comparison of approaches to the instrumental response function in fluorescence decay measurements. Anal Biochem. 1986 Jan;152(1):6–21. doi: 10.1016/0003-2697(86)90111-9. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Cherek H., Balter A. Correction of timing errors in photomultiplier tubes used in phase-modulation fluorometry. J Biochem Biophys Methods. 1981 Sep;5(3):131–146. doi: 10.1016/0165-022x(81)90012-9. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Laczko G., Gryczynski I. Picosecond resolution of tyrosine fluorescence and anisotropy decays by 2-GHz frequency-domain fluorometry. Biochemistry. 1987 Jan 13;26(1):82–90. doi: 10.1021/bi00375a012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. F/F deconvolution of fluorescence decay data. Anal Biochem. 1984 May 1;138(2):314–318. doi: 10.1016/0003-2697(84)90814-5. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Gross D., Webb W. W., Fewtrell C. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1854–1858. doi: 10.1073/pnas.85.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard P. J., Ryan T. A., Webb W. W., Fewtrell C. Immunoglobulin E receptor cross-linking induces oscillations in intracellular free ionized calcium in individual tumor mast cells. J Biol Chem. 1989 Nov 25;264(33):19730–19739. [PubMed] [Google Scholar]
- Oi V. T., Vuong T. M., Hardy R., Reidler J., Dangle J., Herzenberg L. A., Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984 Jan 12;307(5947):136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- Reidler J., Oi V. T., Carlsen W., Vuong T. M., Pecht I., Herzenberg L. A., Stryer L. Rotational dynamics of monoclonal anti-dansyl immunoglobulins. J Mol Biol. 1982 Jul 15;158(4):739–746. doi: 10.1016/0022-2836(82)90258-3. [DOI] [PubMed] [Google Scholar]
- Rodgers M. A., Firey P. A. Instrumentation for fluorescence microscopy with picosecond time resolution. Photochem Photobiol. 1985 Nov;42(5):613–616. doi: 10.1111/j.1751-1097.1985.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- Velez M., Axelrod D. Polarized fluorescence photobleaching recovery for measuring rotational diffusion in solutions and membranes. Biophys J. 1988 Apr;53(4):575–591. doi: 10.1016/S0006-3495(88)83137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos K., van Hoek A., Visser A. J. Application of a reference convolution method to tryptophan fluorescence in proteins. A refined description of rotational dynamics. Eur J Biochem. 1987 May 15;165(1):55–63. doi: 10.1111/j.1432-1033.1987.tb11193.x. [DOI] [PubMed] [Google Scholar]
- Wahl P. Analysis of fluorescence anisotropy decays by a least square method. Biophys Chem. 1979 Jul;10(1):91–104. doi: 10.1016/0301-4622(79)80009-5. [DOI] [PubMed] [Google Scholar]
- Wassler M., Jonasson I., Persson R., Fries E. Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Release of secretory proteins. Biochem J. 1987 Oct 15;247(2):407–415. doi: 10.1042/bj2470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. M., Barisas B. G. Protein rotational motion in solution measured by polarized fluorescence depletion. Biophys J. 1986 Jul;50(1):41–53. doi: 10.1016/S0006-3495(86)83437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


