Abstract
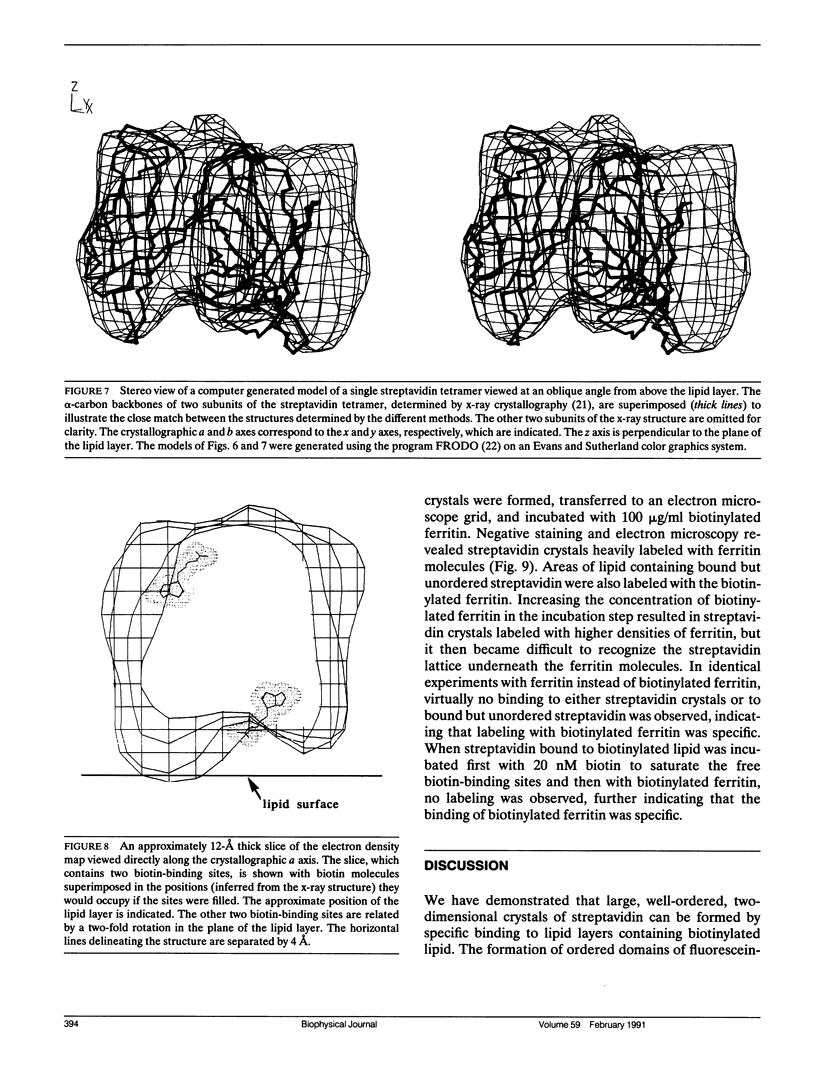
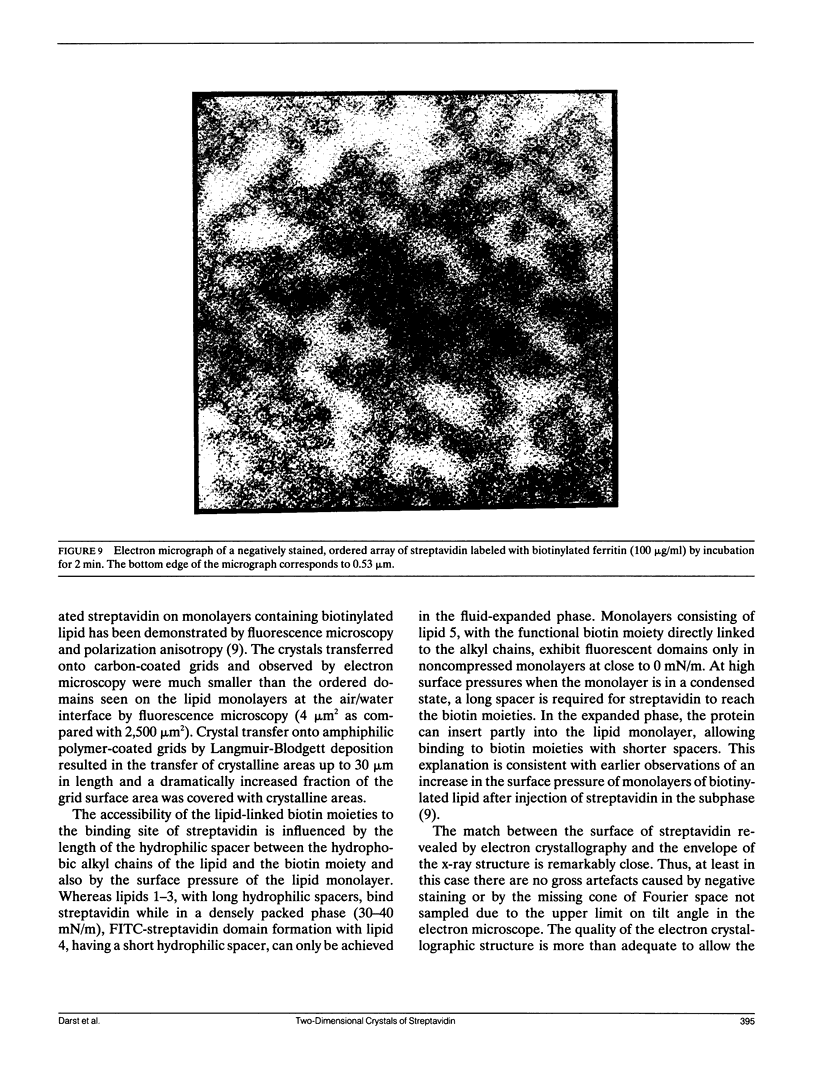
Streptavidin forms two-dimensional crystals when specifically bound to layers of biotinylated lipids at the air/water interface. The three-dimensional structure of streptavidin determined from the crystals by electron crystallography corresponds well with the structure determined by x-ray crystallography. Comparison of the electron and x-ray crystallographic structures reveals the occurrence of free biotin-binding sites on the surface of the two-dimensional crystals facing the aqueous solution. The free biotin-binding sites could be specifically labeled with biotinylated ferritin. The streptavidin/biotinylated lipid system may provide a general approach for the formation of two-dimensional crystals of biotinylated macromolecules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A. A least-squares method for determining structure factors in three-dimensional tilted-view reconstructions. J Mol Biol. 1983 Jul 15;167(4):849–852. doi: 10.1016/s0022-2836(83)80114-4. [DOI] [PubMed] [Google Scholar]
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Blankenburg R., Meller P., Ringsdorf H., Salesse C. Interaction between biotin lipids and streptavidin in monolayers: formation of oriented two-dimensional protein domains induced by surface recognition. Biochemistry. 1989 Oct 3;28(20):8214–8221. doi: 10.1021/bi00446a037. [DOI] [PubMed] [Google Scholar]
- Darst S. A., Ribi H. O., Pierce D. W., Kornberg R. D. Two-dimensional crystals of Escherichia coli RNA polymerase holoenzyme on positively charged lipid layers. J Mol Biol. 1988 Sep 5;203(1):269–273. doi: 10.1016/0022-2836(88)90107-6. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig D. S., Ribi H. O., Schoolnik G. K., Kornberg R. D. Two-dimensional crystals of cholera toxin B-subunit-receptor complexes: projected structure at 17-A resolution. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8585–8588. doi: 10.1073/pnas.83.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Tamm L. K., Weis R. M. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci U S A. 1984 May;81(10):3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargessi R. D., Smith D. S. Fluorometric assays for avidin and biotin. Methods Enzymol. 1986;122:67–72. doi: 10.1016/0076-6879(86)22150-3. [DOI] [PubMed] [Google Scholar]
- Ribi H. O., Reichard P., Kornberg R. D. Two-dimensional crystals of enzyme-effector complexes: ribonucleotide reductase at 18-A resolution. Biochemistry. 1987 Dec 1;26(24):7974–7979. doi: 10.1021/bi00398a064. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E., Kornberg R. D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen--antibody--complement complexes. Nature. 1983 Jan 13;301(5896):125–129. doi: 10.1038/301125a0. [DOI] [PubMed] [Google Scholar]